Answered step by step
Verified Expert Solution
Question
1 Approved Answer
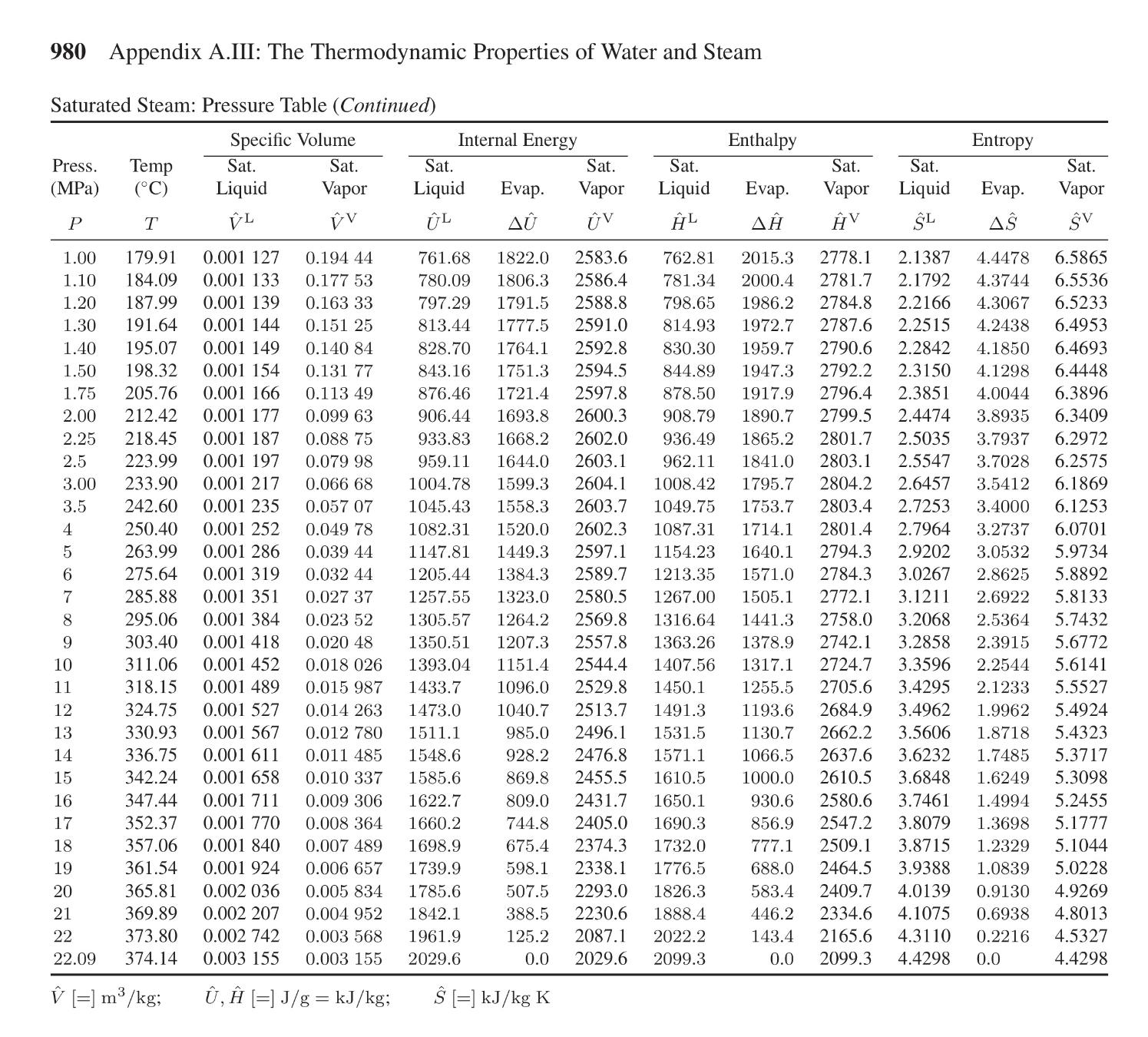
A PVT cell containing pure water ( H 2 O ) shows two equilibrium phases of vapor and liquid at 3 3 6 . 7
A PVT cell containing pure water shows two equilibrium phases of vapor and liquid at Answer the questions below by using Appendix AIll of Sandler, "steam table."
a What is the pressure MPa of the PVT cell?
b The visual window of the PVT cell shows of vapor and of liquid at What is the mass of water a total of liquid water and vapor water in the PVT cell?
c Since the water in the PVT cell shows two equilibrium phases at the temperature and the corresponding pressure you answered in part a it has no thermodynamic freedom When an additional heat is given to the system while keeping the temperature at and the pressure that you answered in part a the volume increases from to Note that the system is closed; hence, the total mass of water remains the same as Part bWhat is the mass of liquid water and what is the mass of vapor water
i got a MPa b g
Can you do part c and check a and b please
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


