Question: A radioactive sample contains two isotopes, A and B. Isotope A decays to isotope B with a half life of t1/2. Isotope B is
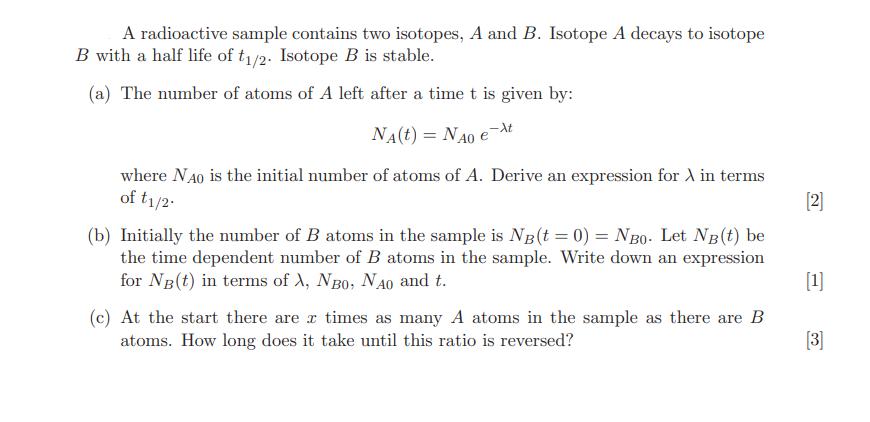
A radioactive sample contains two isotopes, A and B. Isotope A decays to isotope B with a half life of t1/2. Isotope B is stable. (a) The number of atoms of A left after a time t is given by: NA(t) = NAO ext where NO is the initial number of atoms of A. Derive an expression for in terms of t1/2- (b) Initially the number of B atoms in the sample is NB(t = 0) = NBO- Let NB(t) be the time dependent number of B atoms in the sample. Write down an expression for NB(t) in terms of X, NBO, NAO and t. I (c) At the start there are a times as many A atoms in the sample as there are B atoms. How long does it take until this ratio is reversed? [2] [1] [3]
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Isotope A decays to isotope 8 with a halb libe of typ of the numbers of atims of A lebt abter a time ... View full answer

Get step-by-step solutions from verified subject matter experts


