Question
A. Rank the following compounds in order of decreasingacidity, putting the most acidic first. why? CH 3 OCH 3 CH3CHO CH 3 CH2OH CH 3
A. Rank the following compounds in order of decreasingacidity, putting the most acidic first. why?
CH3OCH3 CH3CHO CH3CH2OH CH3COOH
I II III IV
B. Which of the following matches the molecules to thenames of the functional groups? why?
CH3OCH3 Ether
CH3CONH2 Amine
CH3SH Thiol
CH3CHO Alcohol
C. Which of the following compounds has the highestboiling point? why?
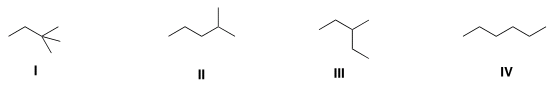
D. Which of the following molecules are chiral?why?
I. 2-Chlorobutane
II. 3-Bromopentane
III. 2-Methylpropene
IV. 2-Bromo-3-methylbutane
E. What is the relationship between the following 2components?? why?
![]()
| Constitutional isomers |
| Identical |
| Stereoisomers |
| Not isomers, different compounds |
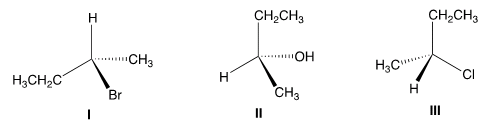
F. Which of the following has the S Configuration?Why?
G. What kind of reaction does theconversion of A to B represent?
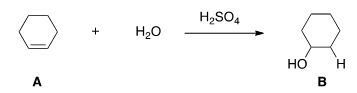
| Acid-base reaction. |
| Elimination reaction. |
| Substitution reaction. |
| Addition reaction. |
H. For the following reaction, usethe identity of the alkyl halide and nucleophile to determine whichsubstitution mechanism occurs. Then determine which solvent affordsthe faster reaction.
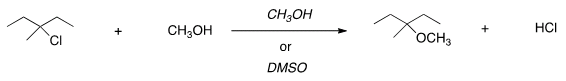
| SN1, CH3OH |
| SN1, DMSO |
| SN2, CH3OH |
| SN2, DMSO |
I. Which of the following is most likely to reactas a nucleophile rather a base?
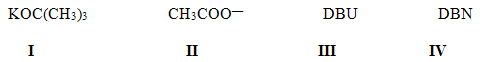
J. What is the most likely mechanism for thereaction below?
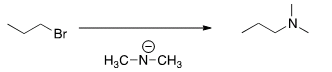
| SN1 |
| SN2 |
| E1 |
| E2 |
What is the best choice of reagent to accomplish thefollowing transformation?

| HCl |
| SOCl2, pyridine |
| Cl2 |
| H3O+, H2O |
|| E III IV
Step by Step Solution
3.30 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below A Rank the following compounds in order of decreasingacidity putting the most acidic first why CH 3 OCH 3 CH3CHO CH 3 CH2OH CH 3 COOH I II ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


