Answered step by step
Verified Expert Solution
Question
1 Approved Answer
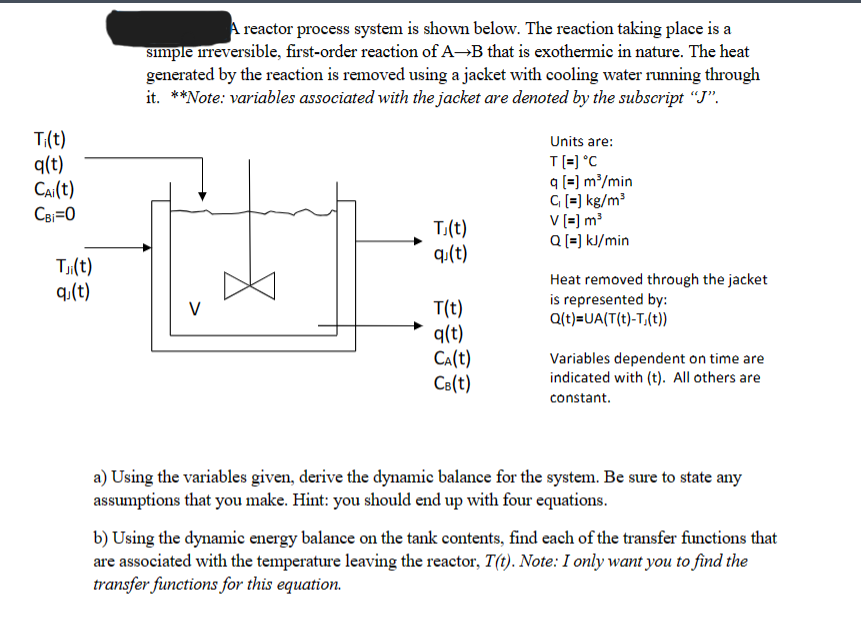
A reactor process system is shown below. The reaction taking place is a simple irreversible, first - order reaction of A B that is exothermic
A reactor process system is shown below. The reaction taking place is a
simple irreversible, firstorder reaction of that is exothermic in nature. The heat
generated by the reaction is removed using a jacket with cooling water running through
itote: variables associated with the jacket are denoted by the subscript
Units are:
Heat removed through the jacket
is represented by:
Variables dependent on time are
indicated with All others are
constant.
a Using the variables given, derive the dynamic balance for the system. Be sure to state any
assumptions that you make. Hint: you should end up with four equations.
b Using the dynamic energy balance on the tank contents, find each of the transfer functions that
are associated with the temperature leaving the reactor, Note: I only want you to find the
transfer functions for this equation. PLEASE DO PART B
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


