Question: A redox buffer contains an oxidizing agent and its conjugate reducing agent. Calculate the potential of a solution containing 0.010 mol of Fe3+ and
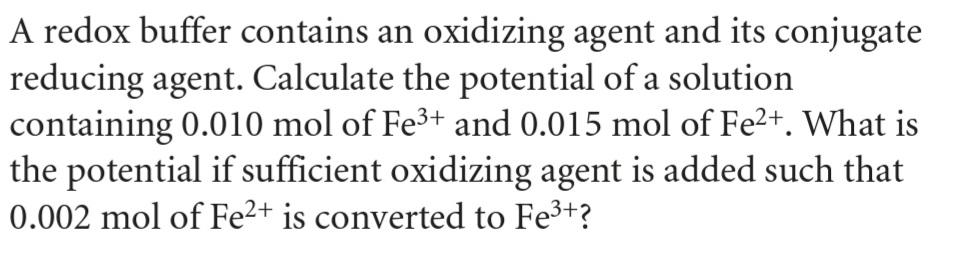
A redox buffer contains an oxidizing agent and its conjugate reducing agent. Calculate the potential of a solution containing 0.010 mol of Fe3+ and 0.015 mol of Fe2+. What is the potential if sufficient oxidizing agent is added such that 0.002 mol of Fe2+ is converted to Fe3+?
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


