Answered step by step
Verified Expert Solution
Question
1 Approved Answer
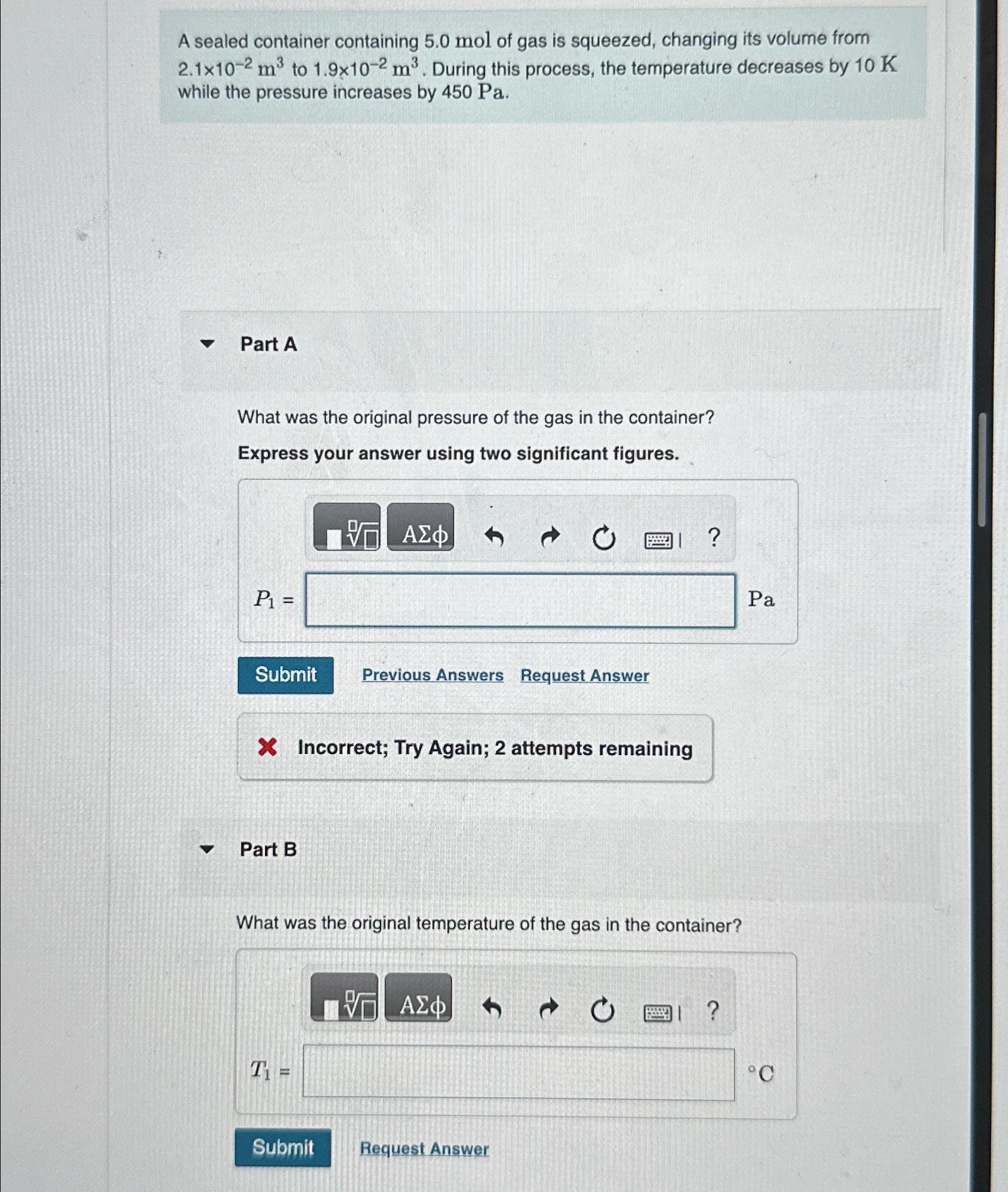
A sealed container containing 5 . 0 mol of gas is squeezed, changing its volume from 2 . 1 1 0 - 2 m 3
A sealed container containing mol of gas is squeezed, changing its volume from to During this process, the temperature decreases by while the pressure increases by
Part A
What was the original pressure of the gas in the container?
Express your answer using two significant figures.
Previous Answers
Request Answer
Incorrect; Try Again; attempts remaining
Part B
What was the original temperature of the gas in the container?
Request Answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


