Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A solid sphere (of radius R and density ) made of substance A (of molecular weight M ) is suspended in liquid B. Solid A
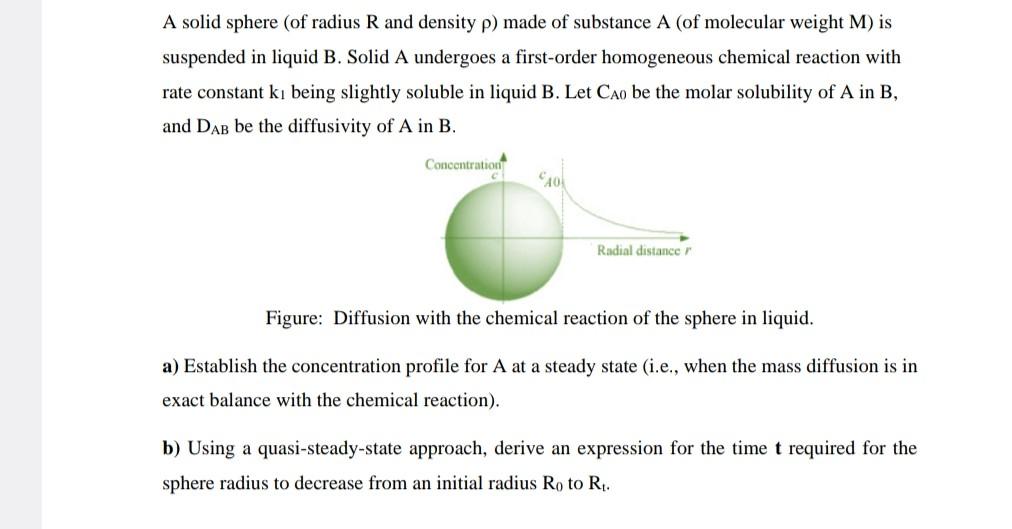
A solid sphere (of radius R and density ) made of substance A (of molecular weight M ) is suspended in liquid B. Solid A undergoes a first-order homogeneous chemical reaction with rate constant k1 being slightly soluble in liquid B. Let CA0 be the molar solubility of A in B, and DAB be the diffusivity of A in B. Figure: Diffusion with the chemical reaction of the sphere in liquid. a) Establish the concentration profile for A at a steady state (i.e., when the mass diffusion is in exact balance with the chemical reaction). b) Using a quasi-steady-state approach, derive an expression for the time t required for the sphere radius to decrease from an initial radius R0 to Rt
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


