Answered step by step
Verified Expert Solution
Question
1 Approved Answer
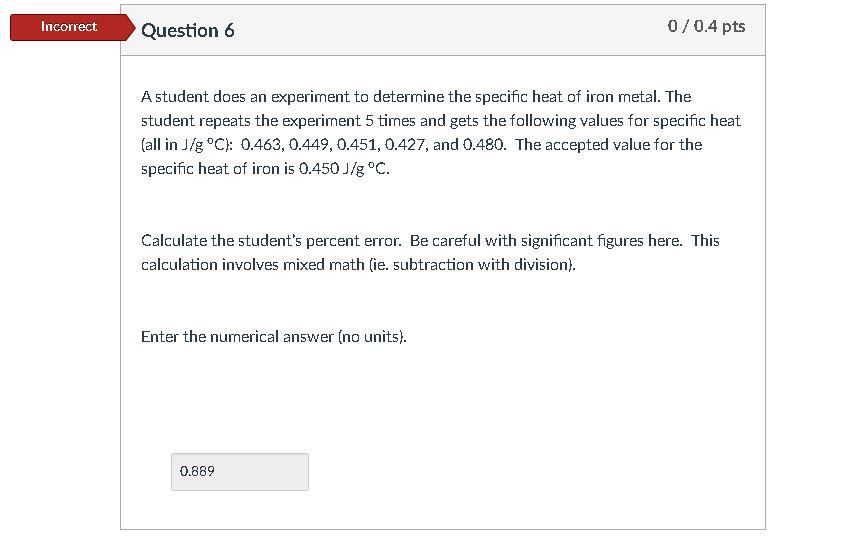
A student does an experiment to determine the specific heat of the iron metal. The student repeats the experiment 5 times and gets the following
A student does an experiment to determine the specific heat of the iron metal. The student repeats the experiment 5 times and gets the following values for specific heat (all in J/g oC): 0.463, 0.449, 0.451, 0.427, and 0.480. The accepted value for the specific heat of iron is 0.450 J/g oC. Calculate the students percent error. Be careful with significant figures here. This calculation involves mixed math (ie. subtraction with division). Enter the numerical answer (no units).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


