Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) The gas constant is 8.31J/molK. The values of D0 and Qd for the diffusion of magnesium in aluminum are 1.2104m2/s and 130kJ/mol, respectively. Compute
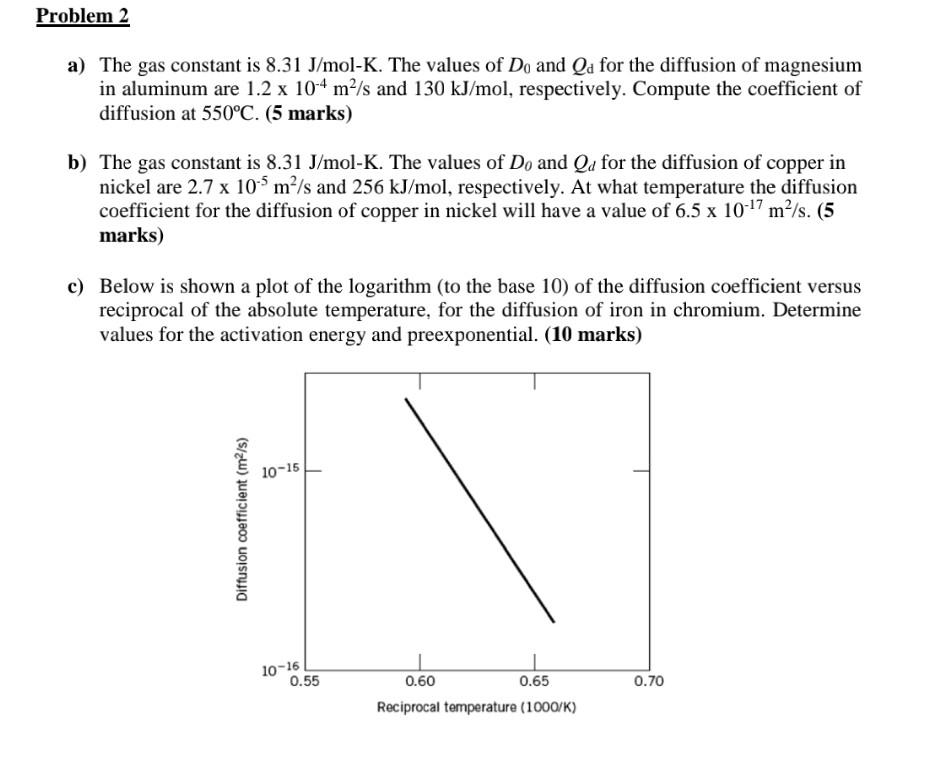
a) The gas constant is 8.31J/molK. The values of D0 and Qd for the diffusion of magnesium in aluminum are 1.2104m2/s and 130kJ/mol, respectively. Compute the coefficient of diffusion at 550C. (5 marks) b) The gas constant is 8.31J/molK. The values of D0 and Qd for the diffusion of copper in nickel are 2.7105m2/s and 256kJ/mol, respectively. At what temperature the diffusion coefficient for the diffusion of copper in nickel will have a value of 6.51017m2/s. (5 marks) c) Below is shown a plot of the logarithm (to the base 10) of the diffusion coefficient versus reciprocal of the absolute temperature, for the diffusion of iron in chromium. Determine values for the activation energy and preexponential. (10 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


