Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A to C Acetylene can be produced from methane using a process flow sheet similar to that sketched below. In this case, the feed contains
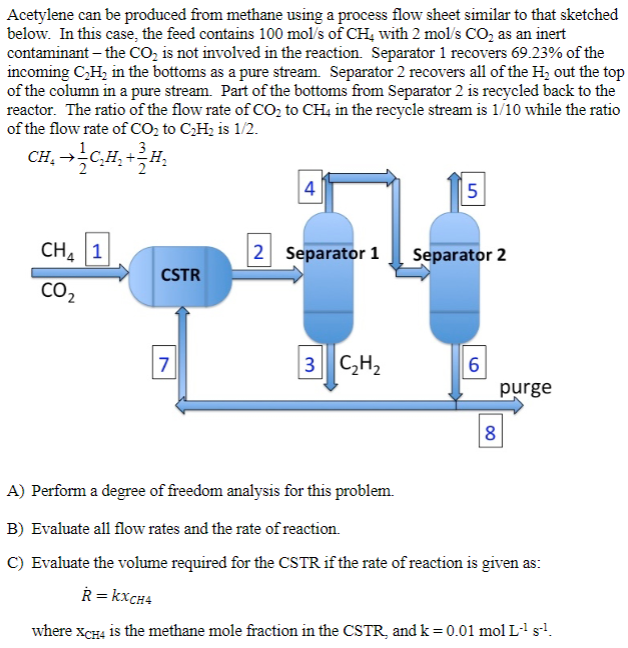
A to C
Acetylene can be produced from methane using a process flow sheet similar to that sketched below. In this case, the feed contains 100mol/s of CH4 with 2mol/sCO2 as an inert contaminant - the CO2 is not involved in the reaction. Separator 1 recovers 69.23% of the incoming C2H2 in the bottoms as a pure stream. Separator 2 recovers all of the H2 out the top of the column in a pure stream. Part of the bottoms from Separator 2 is recycled back to the reactor. The ratio of the flow rate of CO2 to CH4 in the recycle stream is 1/10 while the ratio of the flow rate of CO2 to C2H2 is 1/2. A) Perform a degree of freedom analysis for this problem. B) Evaluate all flow rates and the rate of reaction. C) Evaluate the volume required for the CSTR if the rate of reaction is given as: R=kxCH4 where xCH4 is the methane mole fraction in the CSTR, and k=0.01molL1s1Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


