Answered step by step
Verified Expert Solution
Question
1 Approved Answer
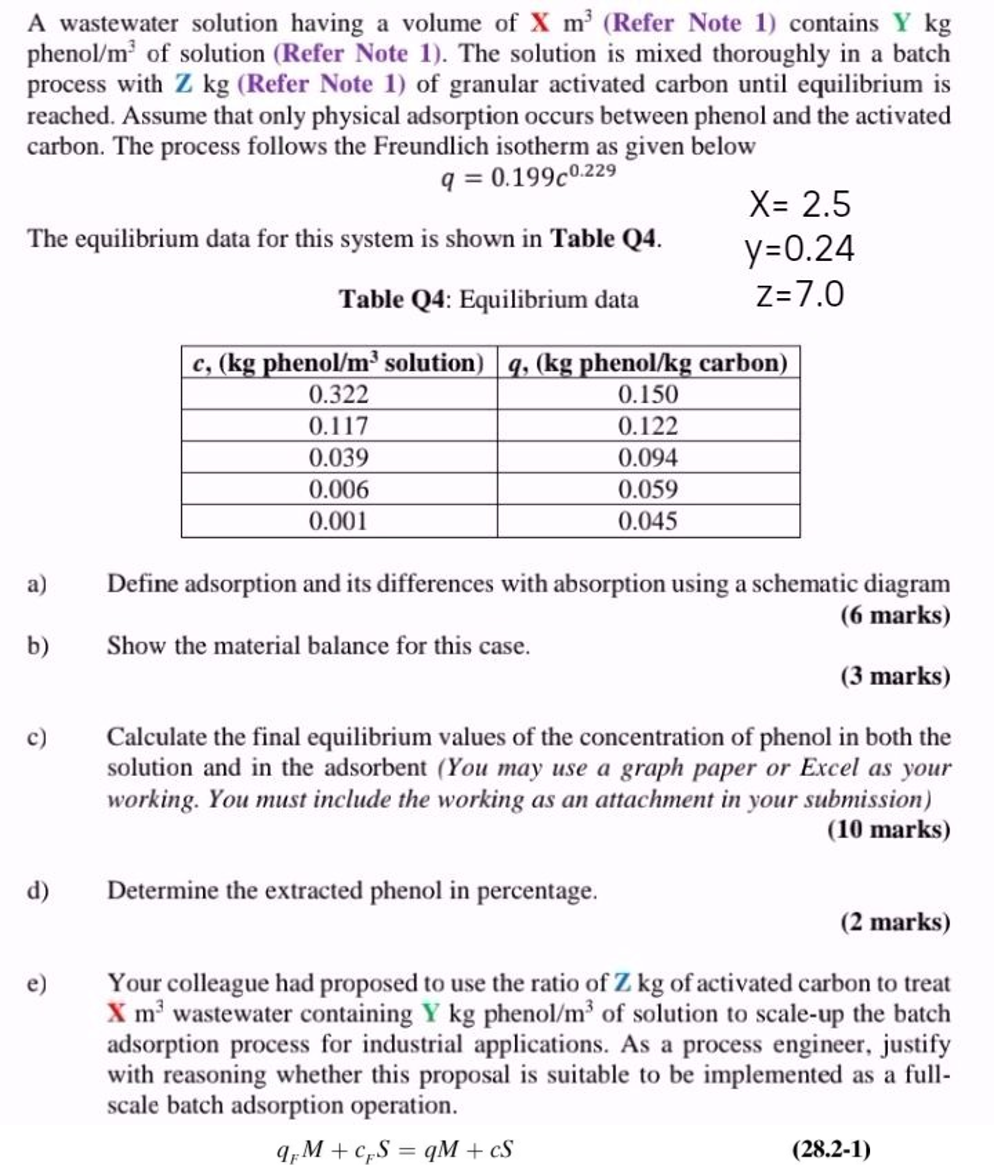
A wastewater solution having a volume of x m 3 ( Refer Note 1 ) contains Y k g phenol ? m 3 of solution
A wastewater solution having a volume of Refer Note contains
phenol of solution Refer Note The solution is mixed thoroughly in a batch
process with Refer Note of granular activated carbon until equilibrium is
reached. Assume that only physical adsorption occurs between phenol and the activated
carbon. The process follows the Freundlich isotherm as given below
The equilibrium data for this system is shown in Table Q
a Define adsorption and its differences with absorption using a schematic diagram
marks
b Show the material balance for this case.
marks
c Calculate the final equilibrium values of the concentration of phenol in both the
solution and in the adsorbent You may use a graph paper or Excel as your
working. You must include the working as an attachment in your submission
marks
d Determine the extracted phenol in percentage.
marks
e Your colleague had proposed to use the ratio of of activated carbon to treat
wastewater containing phenol of solution to scaleup the batch
adsorption process for industrial applications. As a process engineer, justify
with reasoning whether this proposal is suitable to be implemented as a full
scale batch adsorption operation.
use this equation for mass balance
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


