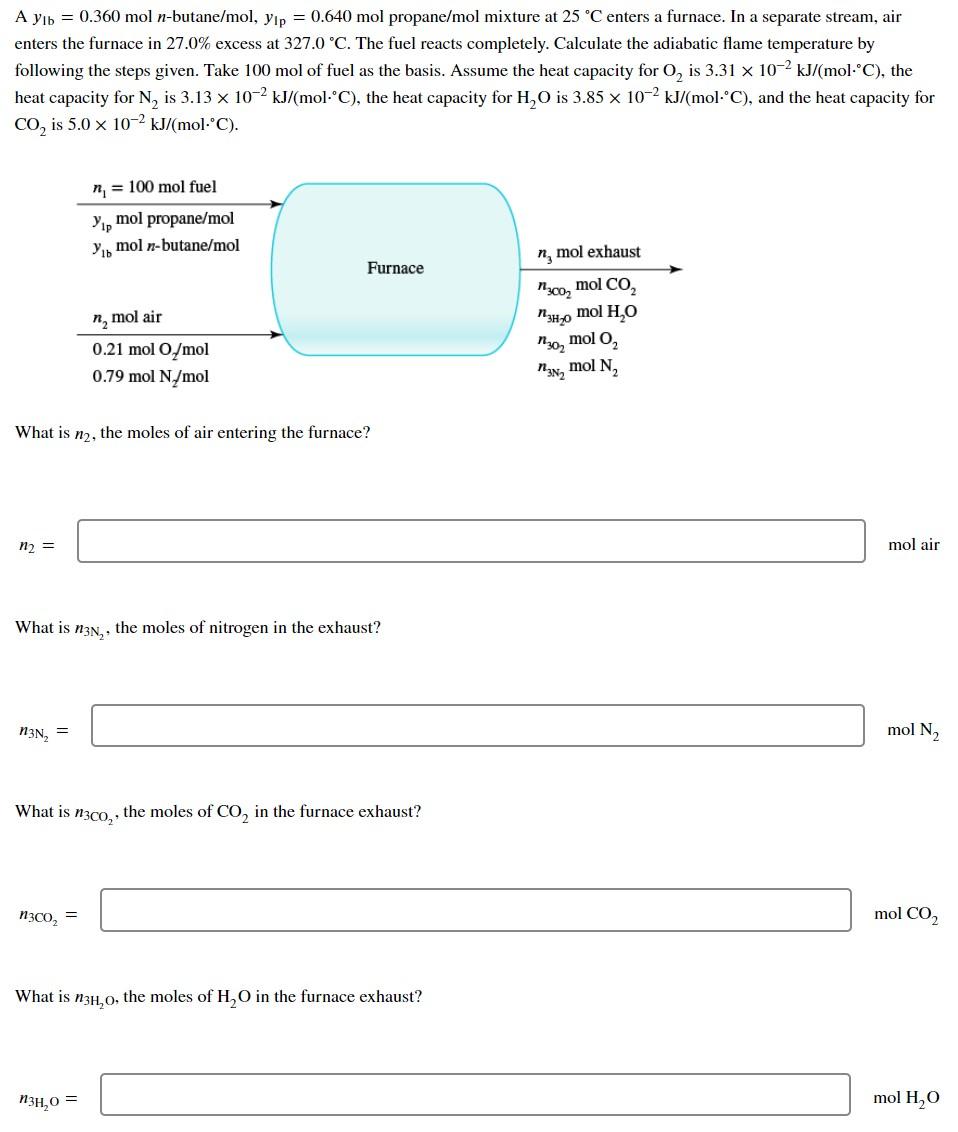


A yb = 0.360 mol n-butane/mol, yp = 0.640 mol propane/mol mixture at 25 C enters a furnace. In a separate stream, air enters the furnace in 27.0% excess at 327.0 C. The fuel reacts completely. Calculate the adiabatic flame temperature by following the steps given. Take 100 mol of fuel as the basis. Assume the heat capacity for 0, is 3.31 x 10-2 kJ/(mol-'C), the heat capacity for N, is 3.13 x 10-2 kJ/(mol-'C), the heat capacity for H,O is 3.85 x 10-2 kJ/(mol-'C), and the heat capacity for CO, is 5.0 x 10-2 kJ/(mol.C). n = 100 mol fuel Yo mol propane/mol Yib mol n-butane/mol ng mol exhaust Furnace n, mol air :co, mol CO, 3,0 mol H2O mol O2 mol N , 0.21 mol O/mol 0.79 mol N/mol , What is n2, the moles of air entering the furnace? n2 = mol air What is n3N,, the moles of nitrogen in the exhaust? = n3N, mol N2 What is n3co,, the moles of Co, in the furnace exhaust? n3co, = mol CO2 What is n34,0, the moles of H2O in the furnace exhaust? ,0 = mol H, What is n30, the moles of O, in the furnace exhaust? n302 mol O2 Use the heat of formation method for all of the remaining calculations, and refer to the table and reference states. References: C(s), H2(g), O2(g), and N, (g) at 25C at 1 atm Substance Nin (mol) Hin (kJ/mol) CH nout Hout (kJ/mol) MICH n-Catho NCH HICH HICHO HN H 20 n2N, N3N H3N 120, 130, H 30, H, N2 02 CO2 n3co H2O ,0 What is H CH, the specific enthalpy of propane entering the furnace? H HICH = kJ/mol What is Chy, the specific enthalpy of n-butane entering the furnace? HICHO = kJ/mol What is H 2N, the specific enthalpy of N, entering the furnace? HN = kJ/mol What is 20,, the specific enthalpy of O, entering the furnace? 20,= = kJ/mol What is Tab, the adiabatic flame temperature of the process? Tab = C A yb = 0.360 mol n-butane/mol, yp = 0.640 mol propane/mol mixture at 25 C enters a furnace. In a separate stream, air enters the furnace in 27.0% excess at 327.0 C. The fuel reacts completely. Calculate the adiabatic flame temperature by following the steps given. Take 100 mol of fuel as the basis. Assume the heat capacity for 0, is 3.31 x 10-2 kJ/(mol-'C), the heat capacity for N, is 3.13 x 10-2 kJ/(mol-'C), the heat capacity for H,O is 3.85 x 10-2 kJ/(mol-'C), and the heat capacity for CO, is 5.0 x 10-2 kJ/(mol.C). n = 100 mol fuel Yo mol propane/mol Yib mol n-butane/mol ng mol exhaust Furnace n, mol air :co, mol CO, 3,0 mol H2O mol O2 mol N , 0.21 mol O/mol 0.79 mol N/mol , What is n2, the moles of air entering the furnace? n2 = mol air What is n3N,, the moles of nitrogen in the exhaust? = n3N, mol N2 What is n3co,, the moles of Co, in the furnace exhaust? n3co, = mol CO2 What is n34,0, the moles of H2O in the furnace exhaust? ,0 = mol H, What is n30, the moles of O, in the furnace exhaust? n302 mol O2 Use the heat of formation method for all of the remaining calculations, and refer to the table and reference states. References: C(s), H2(g), O2(g), and N, (g) at 25C at 1 atm Substance Nin (mol) Hin (kJ/mol) CH nout Hout (kJ/mol) MICH n-Catho NCH HICH HICHO HN H 20 n2N, N3N H3N 120, 130, H 30, H, N2 02 CO2 n3co H2O ,0 What is H CH, the specific enthalpy of propane entering the furnace? H HICH = kJ/mol What is Chy, the specific enthalpy of n-butane entering the furnace? HICHO = kJ/mol What is H 2N, the specific enthalpy of N, entering the furnace? HN = kJ/mol What is 20,, the specific enthalpy of O, entering the furnace? 20,= = kJ/mol What is Tab, the adiabatic flame temperature of the process? Tab = C









