Question
Complete the calculations Trial 1 Trial 2 99.612 79.023 Mass of Water 3.702 52.230 Mass of NaOH(s) 103.314 g 131.253 g Total Mass in Cup
Complete the calculations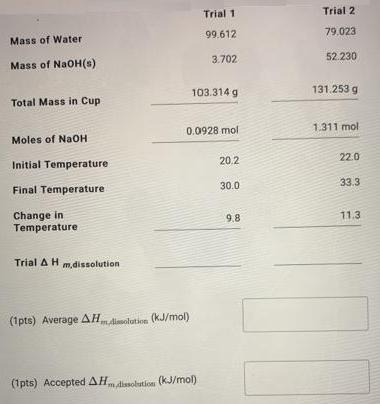
Trial 1 Trial 2 99.612 79.023 Mass of Water 3.702 52.230 Mass of NaOH(s) 103.314 g 131.253 g Total Mass in Cup 1.311 mol 0.0928 mol Moles of NaOH 20.2 22.0 Initial Temperature 30.0 33.3 Final Temperature Change in Temperature 9.8 11.3 Trial AH m,dissolution (1pts) Average AHAlsolation (kJ/mol) (1pts) Accepted AH mdissolution (kJ/mol)
Step by Step Solution
3.45 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
when we add NaOH exothermic reaction occur then we have to find how much heat is absorbed by solutio...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Vector Mechanics for Engineers Statics and Dynamics
Authors: Ferdinand Beer, E. Russell Johnston Jr., David Mazurek, Phillip Cornwell, Brian Self
11th edition
73398241, 978-0073398242
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



