Question
A+BC+2D reaction was carried out in a differential reactor and the following data were obtained. a) Develop a rate law and find all the constants
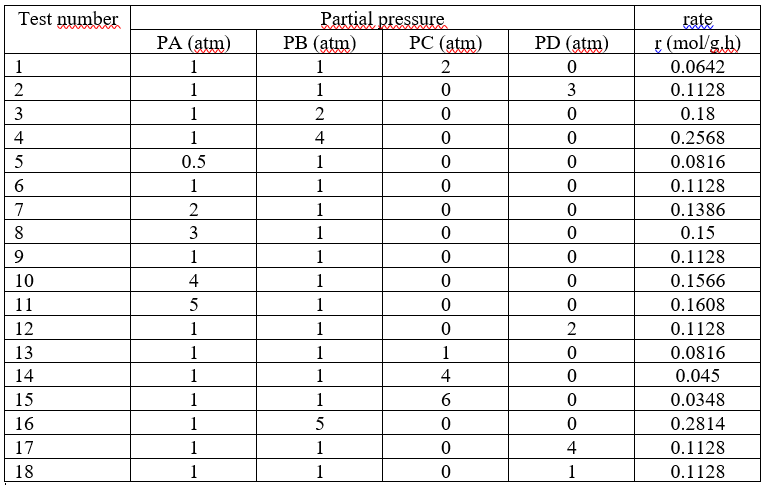
A+BC+2D reaction was carried out in a differential reactor and the following data were obtained.
a) Develop a rate law and find all the constants in the rate law.
b) Suggest a mechanism suitable for the experimental data and show the compatibility of the velocity expression you will obtain from the mechanism with the velocity expression you will consider from the experimental data. Specify the check digit.
c) Calculate the catalyst weight required to reach 80% conversion in a Continuous Stirred Tank Reactor (SKTR) since A and B are fed at 3 atm at 200 oC at stoichiometric ratios of 5 mol/min in total.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


