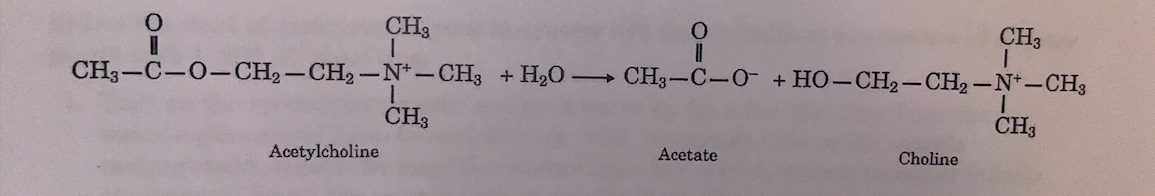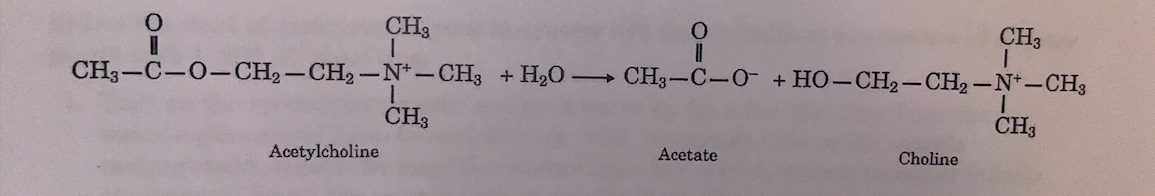


Acetylcholine Acetate Choline POST-LAB QUESTIONS 1. Calculate the enzyme activities with acetylcholine as the aubatrate for both eaxymes. Using the change in absorbencies between 30 sec. and 2min., caleulate the rate of reaction as change () in absorbance/min. for a. acetylcholine esterase b. carboxyl esterase c. You have calculated the amount of acetylcholine eaterase that you had in the sample tube (Pre-Lab Question no. 3). Use this number to calculate the activity of this eazyme toward acetyleholine in A absorbenco/min/mg enzyme. d. You have calculated the amount of carboxyl esternse that you had in the sample tube (Pre-Lab Question no. 4). Uee this number to calculate the activity of this enzyme toward acetyleholine in A absorbence/min/mg enzyme. e. Which enzyme catalyzes the hydrolysis of acetylcholine fnater? How many times fastor? 2. Calculate the enzyme nctivities of o-nitropheayl acetate as a substrate for both enzymes. Using the change in abeorbence between 30 see. and 2min. in the reaction, calculate the rate of reaction toward o-nitrophenyl ncetate in absorbence/min. for a. acetylcholine eaterase b. carboxyl esterase c. You have calculated the amount of acetylcholine esterase in your sample tube (Pre-Lab Question no. 3). Use this number to calculate the activity of this ensyme toward o-nitrophenyl acetate in absorbence/min/mg enzyme. d. You have calculated the amount of carboxyl esterase in your sample tube (Pre-Lab Question no. 4). Use this number to calculate the activity of this enzyme toward o.nitrophenyl acetate in absorbenodminimg enzymo. e. Which enzyme catalyzes the hydrolysis of o-aitrophenyl faster? How many times faster? PRE-LAB QUESTIONS 1. Acetylcholize is sold as acetylcholine chloride. Looking at the atructures given in the Background, write the formula for the anion and the cation of this salt. 2. The molecular weight of acetylcholine chloride is 181.7. A stock solutien of 1.817g acetylcholine in 10.0mL was prepared by the stockroom. What is the concentration of this stock solution in % wiv and in molnzity? Froen this stock solution of acetyleholine chloride, 0.40mL was added to the sample tube bringing the total volume in the sample tube to 3.0ml. What is the conoentration of the acetylcholine chloride is the sample tube in gimL and in molarity? 3. Acetylcholine esterase, 3.0mg, was dissolved in 10.0mI solution. From this solution, 0.60mL was added to the sumple tabe, bringing the volume to a tetal of 3.0mL How many mg of acetylcholine eaterase did you have in the aample tabe? 4. The commercial sample of carboxyl esternse contained 15.0mg of enxyme ia 0.30mL of suspension. In your experiment, you add 0.090mL ef this suspension to the sample tube that contains a tetal volume of 9.0mL. How many mg of carboxyl esterase entyme do you have in the sample tube? Acetylcholine Acetate Choline POST-LAB QUESTIONS 1. Calculate the enzyme activities with acetylcholine as the aubatrate for both eaxymes. Using the change in absorbencies between 30 sec. and 2min., caleulate the rate of reaction as change () in absorbance/min. for a. acetylcholine esterase b. carboxyl esterase c. You have calculated the amount of acetylcholine eaterase that you had in the sample tube (Pre-Lab Question no. 3). Use this number to calculate the activity of this eazyme toward acetyleholine in A absorbenco/min/mg enzyme. d. You have calculated the amount of carboxyl esternse that you had in the sample tube (Pre-Lab Question no. 4). Uee this number to calculate the activity of this enzyme toward acetyleholine in A absorbence/min/mg enzyme. e. Which enzyme catalyzes the hydrolysis of acetylcholine fnater? How many times fastor? 2. Calculate the enzyme nctivities of o-nitropheayl acetate as a substrate for both enzymes. Using the change in abeorbence between 30 see. and 2min. in the reaction, calculate the rate of reaction toward o-nitrophenyl ncetate in absorbence/min. for a. acetylcholine eaterase b. carboxyl esterase c. You have calculated the amount of acetylcholine esterase in your sample tube (Pre-Lab Question no. 3). Use this number to calculate the activity of this ensyme toward o-nitrophenyl acetate in absorbence/min/mg enzyme. d. You have calculated the amount of carboxyl esterase in your sample tube (Pre-Lab Question no. 4). Use this number to calculate the activity of this enzyme toward o.nitrophenyl acetate in absorbenodminimg enzymo. e. Which enzyme catalyzes the hydrolysis of o-aitrophenyl faster? How many times faster? PRE-LAB QUESTIONS 1. Acetylcholize is sold as acetylcholine chloride. Looking at the atructures given in the Background, write the formula for the anion and the cation of this salt. 2. The molecular weight of acetylcholine chloride is 181.7. A stock solutien of 1.817g acetylcholine in 10.0mL was prepared by the stockroom. What is the concentration of this stock solution in % wiv and in molnzity? Froen this stock solution of acetyleholine chloride, 0.40mL was added to the sample tube bringing the total volume in the sample tube to 3.0ml. What is the conoentration of the acetylcholine chloride is the sample tube in gimL and in molarity? 3. Acetylcholine esterase, 3.0mg, was dissolved in 10.0mI solution. From this solution, 0.60mL was added to the sumple tabe, bringing the volume to a tetal of 3.0mL How many mg of acetylcholine eaterase did you have in the aample tabe? 4. The commercial sample of carboxyl esternse contained 15.0mg of enxyme ia 0.30mL of suspension. In your experiment, you add 0.090mL ef this suspension to the sample tube that contains a tetal volume of 9.0mL. How many mg of carboxyl esterase entyme do you have in the sample tube