Answered step by step
Verified Expert Solution
Question
1 Approved Answer
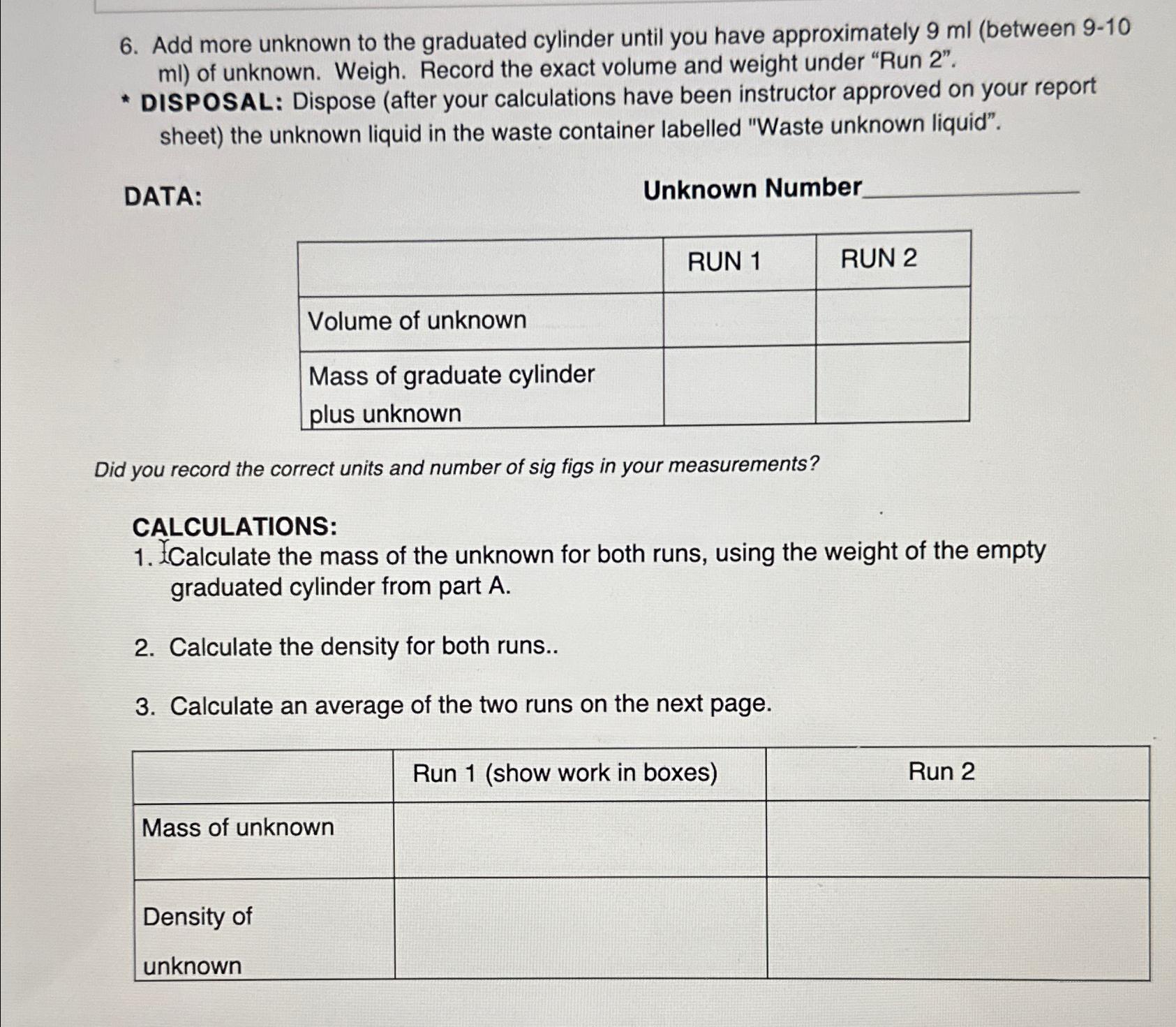
Add more unknown to the graduated cylinder until you have approximately 9 m l ( between 9 - 1 0 m l ) of unknown.
Add more unknown to the graduated cylinder until you have approximately between of unknown. Weigh. Record the exact volume and weight under "Run
DISPOSAL: Dispose after your calculations have been instructor approved on your report sheet the unknown liquid in the waste container labelled "Waste unknown liquid".
DATA:
Unknown Number
tableRUN RUN Volume of unknown,,tableMass of graduate cylinderplus unknown
Did you record the correct units and number of sig figs in your measurements?
CALCULATIONS:
XCalculate the mass of the unknown for both runs, using the weight of the empty graduated cylinder from part
Calculate the density for both runs..
Calculate an average of the two runs on the next page.
tableRun show work in boxesRun Mass of unknown,,tableDensity ofunknown
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


