Question
Adenosine triphosphate is composed of the nitrogenous base adenine, the five-carbon sugar ribose, and three phosphate groups. Then calculate the free energy of ATP
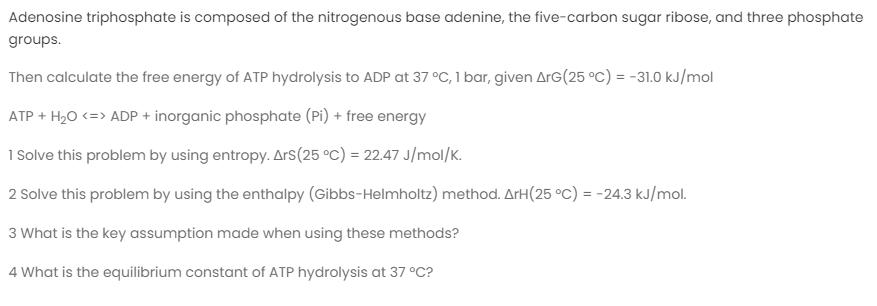
Adenosine triphosphate is composed of the nitrogenous base adenine, the five-carbon sugar ribose, and three phosphate groups. Then calculate the free energy of ATP hydrolysis to ADP at 37 C, 1 bar, given ArG(25 C) = -31.0 kJ/mol ATP + H20 ADP + inorganic phosphate (Pi) + free energy 1 Solve this problem by using entropy. Ars(25 C) = 22.47 J/mol/K. 2 Solve this problem by using the enthalpy (Gibbs-Helmholtz) method. ArH(25 C) = -24.3 kJ/mol. 3 What is the key assumption made when using these methods? 4 What is the equilibrium constant of ATP hydrolysis at 37 C?
Step by Step Solution
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
1 using a AG ts AS 3 AT for measurable change we can write AT 325c 12 4625C A0 265...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles of Corporate Finance
Authors: Richard A. Brealey, Stewart C. Myers
7th edition
72869461, 72467665, 9780072467666, 978-0072869460
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



