Answered step by step
Verified Expert Solution
Question
1 Approved Answer
All i need is just answers ! please help from 1 to 7 2. At a certain temperature, the equilibrium constant for the reaction given
All i need is just answers ! please help from 1 to 7 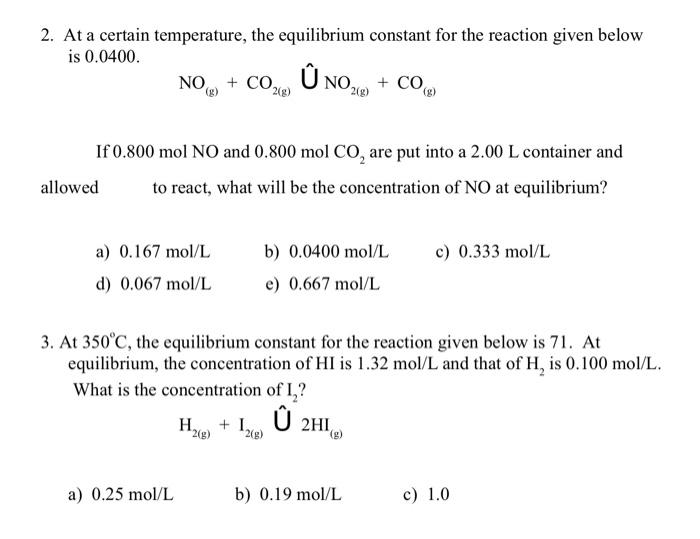


2. At a certain temperature, the equilibrium constant for the reaction given below is 0.0400. NO(g)+CO2(g)U^NO2(g)+CO(g) If 0.800molNO and 0.800molCO2 are put into a 2.00L container and allowed to react, what will be the concentration of NO at equilibrium? a) 0.167mol/L b) 0.0400mol/L c) 0.333mol/L d) 0.067mol/L e) 0.667mol/L 3. At 350C, the equilibrium constant for the reaction given below is 71 . At equilibrium, the concentration of HI is 1.32mol/L and that of H2 is 0.100mol/L. What is the concentration of I2 ? H2(g)+I2(g)U^2HI(g) a) 0.25mol/L b) 0.19mol/L c) 1.0 mol/L d) 0.32mol/L e) 0.57mol/L 4. At 70C, the equilibrium constant for the reaction given below is 0.090. If at equilibrium [N2O4]=3.22mol/L, what is the molar concentration of NO2 ? N2O4(D)2NO2(e) a) 0.54mol/L b) 6.4mol/L c) 6.0mol/L d) 0.29mol/L e) 0.084mol/L 5. Consider the following equilibrium for which K is 125 at 400C. A(w)+Bm)n2AB If 2.00mol of Aavi and 1.00mol of Bxp are placed in a 1.0L container and allowed to react, what will be the concentration of AB at equilibrium? a) 0.791mol/L b) 1.94mol/L c) 0.971mol/L d) 3.50mol/L e) 1.14mol/L was established. Determine the value of K for this reaction if the equilibrium concentrations were: [NOie]=0.060mol/L [N2O(6)]=0.015mol/L [NO2(t)]= 0.025mol/L a) 1.7 b) 0.58 c) 480 d) 6.4103 e) 3.4 x103 PClrls+Cl2(g)PCl5(s) At a certain temperature, 0.500mol of PCl5 was placed into a 0.250L container and allowed to react. At equilibrium, the container held 0.100mol of PCl5. What is the value of K for this reaction? a) 0.100 b) 0.500 c) 0.156 d) 0.625 e) 0.833 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


