Answered step by step
Verified Expert Solution
Question
1 Approved Answer
all questions please 3. What is Charles Law? Boyle's Law? 4. What are the characteristics of an ideal gas? How is it different from a
all questions please
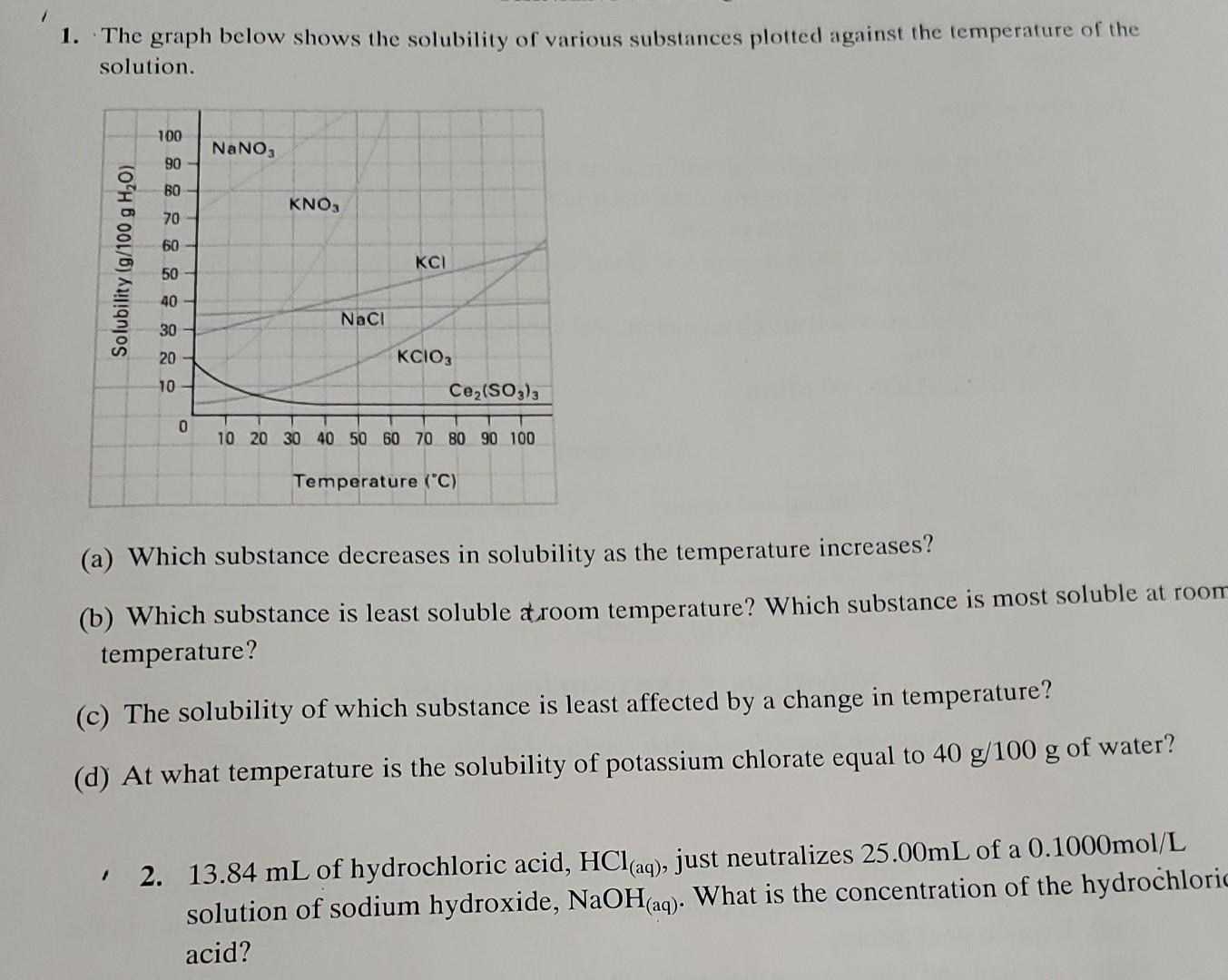
3. What is Charles Law? Boyle's Law? 4. What are the characteristics of an ideal gas? How is it different from a real gas? 1. The graph below shows the solubility of various substances plotted against the temperature of the solution. (a) Which substance decreases in solubility as the temperature increases? (b) Which substance is least soluble aroom temperature? Which substance is most soluble at room temperature? (c) The solubility of which substance is least affected by a change in temperature? (d) At what temperature is the solubility of potassium chlorate equal to 40g/100g of water? 2. 13.84mL of hydrochloric acid, HCl(aq), just neutralizes 25.00mL of a 0.1000mol/L solution of sodium hydroxide, NaOH(aq). What is the concentration of the hydrochloric acidStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


