Question
aluminum hydride: AGO = = AIH3(s) Al(s) + 3/2 H(g) kJ/mol kJ/mol
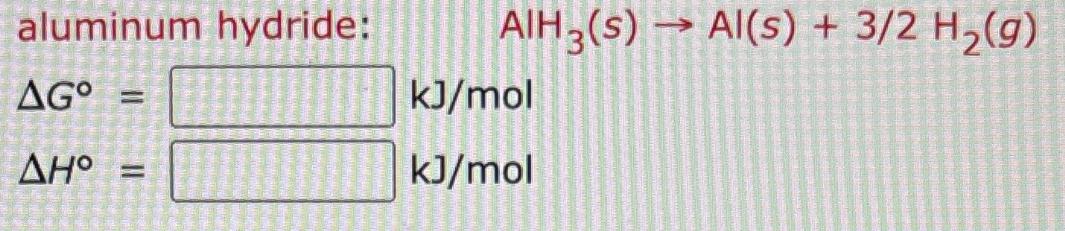
aluminum hydride: AGO = = AIH3(s) Al(s) + 3/2 H(g) kJ/mol kJ/mol
Step by Step Solution
3.46 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
Youve presented an image of a chemical reaction along with two blank boxes asking for the standard G...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles The Quest For Insight
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
7th Edition
1464183953, 9781464183959
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



