Answered step by step
Verified Expert Solution
Question
1 Approved Answer
am uploading a picture of my work. My answer of 27.2kJ/mol was marked wrong. f=0.5Ap=[L](f11)=50pM(i,4,5)[I]=K=50pM Here is a cleaned up version of what you should
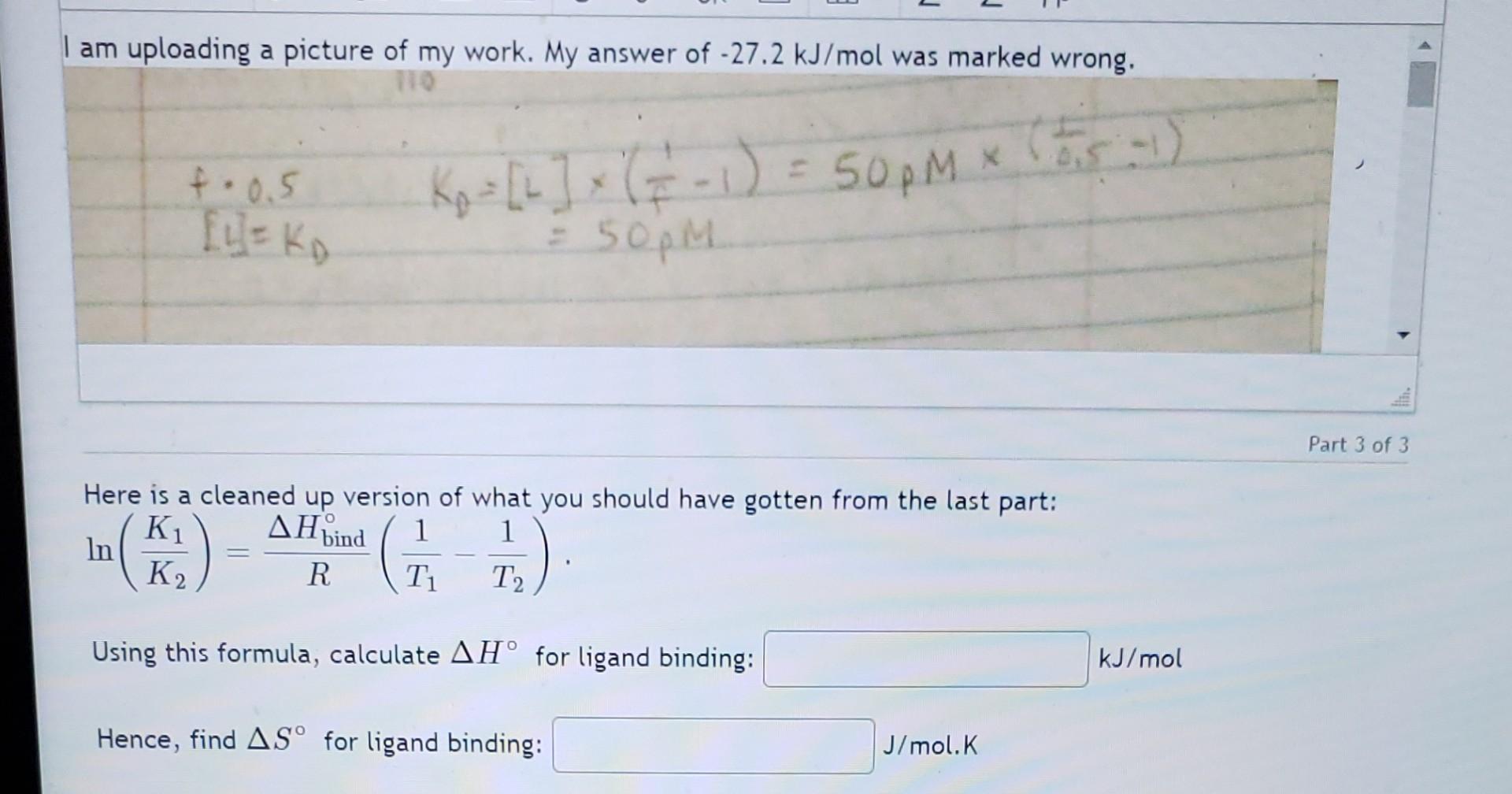
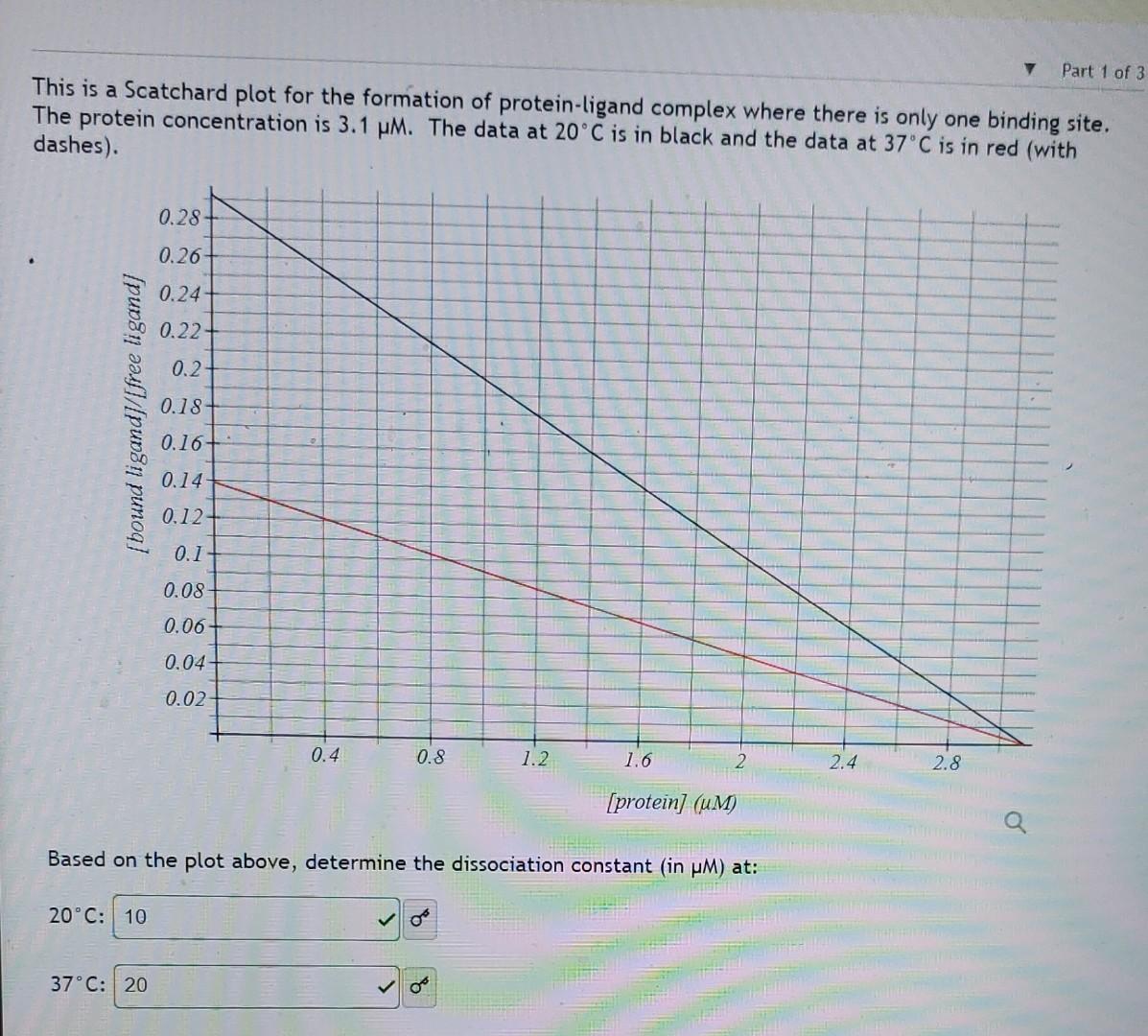

am uploading a picture of my work. My answer of 27.2kJ/mol was marked wrong. f=0.5Ap=[L](f11)=50pM(i,4,5)[I]=K=50pM Here is a cleaned up version of what you should have gotten from the last part: ln(K2K1)=RHbind(T11T21) Using this formula, calculate H for ligand binding: kJ/mol Hence, find S for ligand binding: J/mol.K This is a Scatchard plot for the formation of protein-ligand complex where there is only one binding site. The protein concentration is 3.1M. The data at 20C is in black and the data at 37C is in red (with dashes). Based on the plot above, determine the dissociation constant (in M ) at: 20C : 37C: Part 2 of 3 In this part of the question, we will use the KD values we found to back-derive thermodynamical values. What is Gbind at 37C ? kJ/mol ) of kJ/mol (report your answer to the nearest 0.1 Hint: Remember that Gbind=RTlnKD. From G=HTS and the equation relating K and G, derive an expression relating KD and Hbind (be sure to understand that binding is the reverse of dissociation!). Assume H and S are temperature independent. In this expression: K1 is KD for temperature T1 and K2 is KD for temperature T2. Give the expression here as an equation. You can leave it as any form of equation: ln(K2K1)=(RT1DeltaH)(T21)s Hint: Start by writing an expression for G1G2 and doing the same thing for the expressions of these in terms of their respective KD s. You may wish to consider how you could use the log properties to help you
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


