Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Ammonia is synthesized at 200 bar and 773 K by the reaction: Na +3712 + 2NH3 The yield of ammonia 15 0.45 mol/mol of the
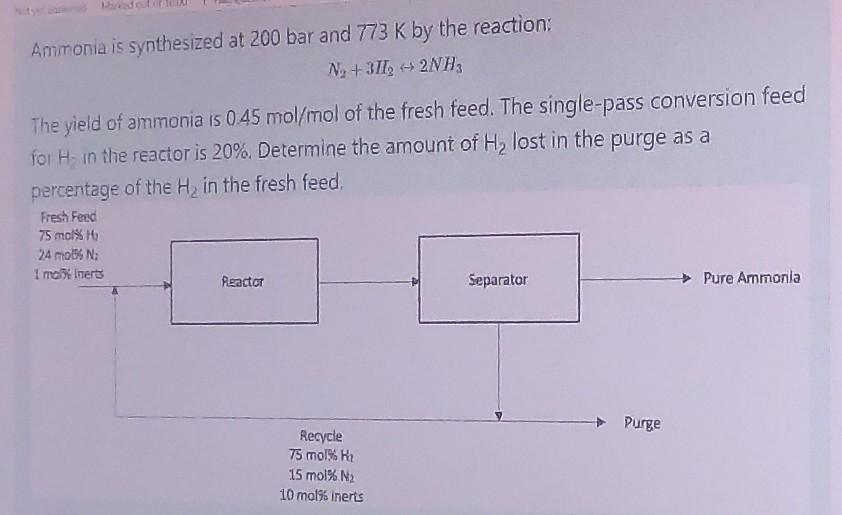
Ammonia is synthesized at 200 bar and 773 K by the reaction: Na +3712 + 2NH3 The yield of ammonia 15 0.45 mol/mol of the fresh feed. The single-pass conversion feed for Hin the reactor is 20%. Determine the amount of Hy lost in the purge as a percentage of the H, in the fresh feed. Fresh Feed 75 mol% 763 24 4 : I moliners Separator Pure Ammonia Peactor Purge Recycle 75 mol% 2 15 mol% N2 10 mol% inerts
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


