Answered step by step
Verified Expert Solution
Question
1 Approved Answer
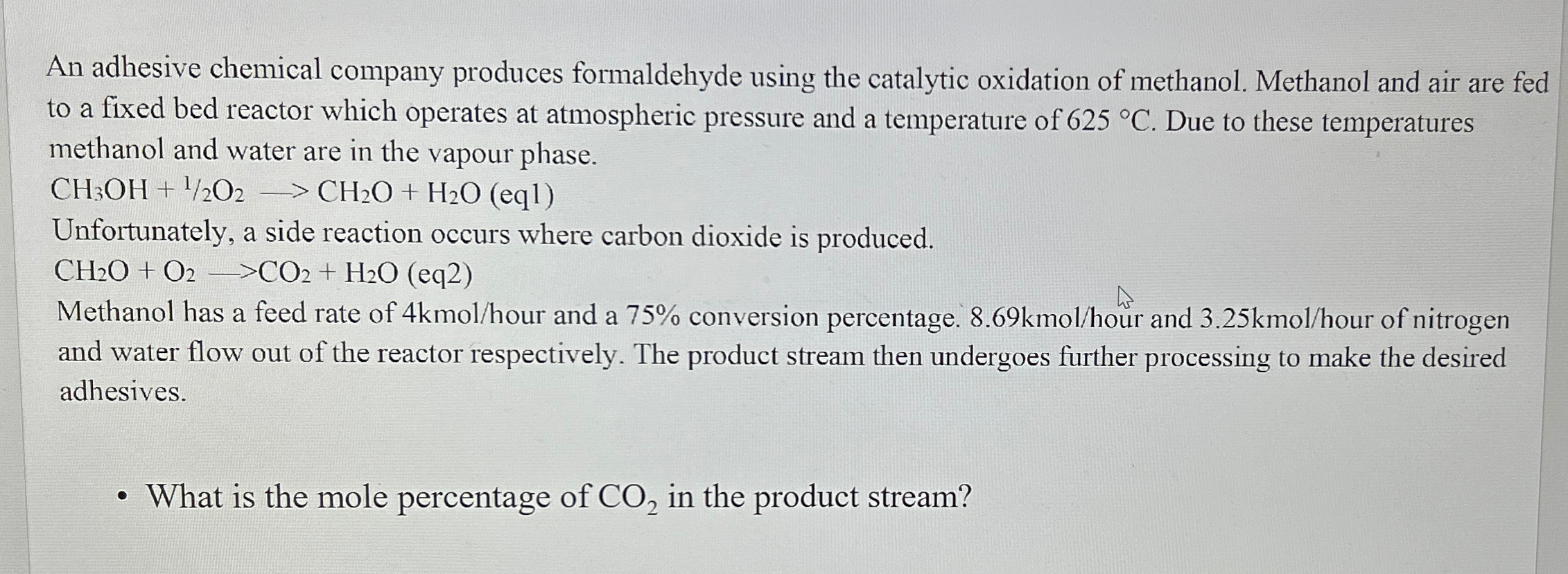
An adhesive chemical company produces formaldehyde using the catalytic oxidation of methanol. Methanol and air are fed to a fixed bed reactor which operates at
An adhesive chemical company produces formaldehyde using the catalytic oxidation of methanol. Methanol and air are fed to a fixed bed reactor which operates at atmospheric pressure and a temperature of Due to these temperatures methanol and water are in the vapour phase.
Unfortunately, a side reaction occurs where carbon dioxide is produced.
Methanol has a feed rate of kmo hour and a conversion percentage. kmo hour and kmoour of nitrogen and water flow out of the reactor respectively. The product stream then undergoes further processing to make the desired adhesives.
What is the mole percentage of in the product stream?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


