Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An average person's lungs contain about 5 L of gas . 1 under normal conditions. If a diver makes a free dive ( no breathing
An average person's lungs contain about of gas under normal conditions. If a diver makes a free dive no breathing apparatus the volume of the lungs is compressed when the pressure equalizes throughout the body. If compression occurs below irreversible lung damage will occur. Calculate the maximum safe depth for a free dive in seawater assume the density is the same as freshwater
What is the density of propane gas in kilograms per cubic meter at kPa and
What is the specific gravity of propane Calculate the number of cubic meters of hydrogen sulfide, measured at a temperature of and a pressure of gauge pressure which may be produced from of iron sulfide
A rigid tank containing hydrogen at and kPa is connected by a valve to another rigid tank that holds hydrogen at and kPa. Now the valve is opened and the system is allowed to reach thermal equilibrium with the surroundings, which are at Determine the final pressure in the tank
Methane is completely burned with excess air, with of the carbon going to What is the partial pressure of the in the stack gas if the barometer reads How many kilograms of can be put into a cylinder at room temperature and kPa absolute pressure? kPaTc
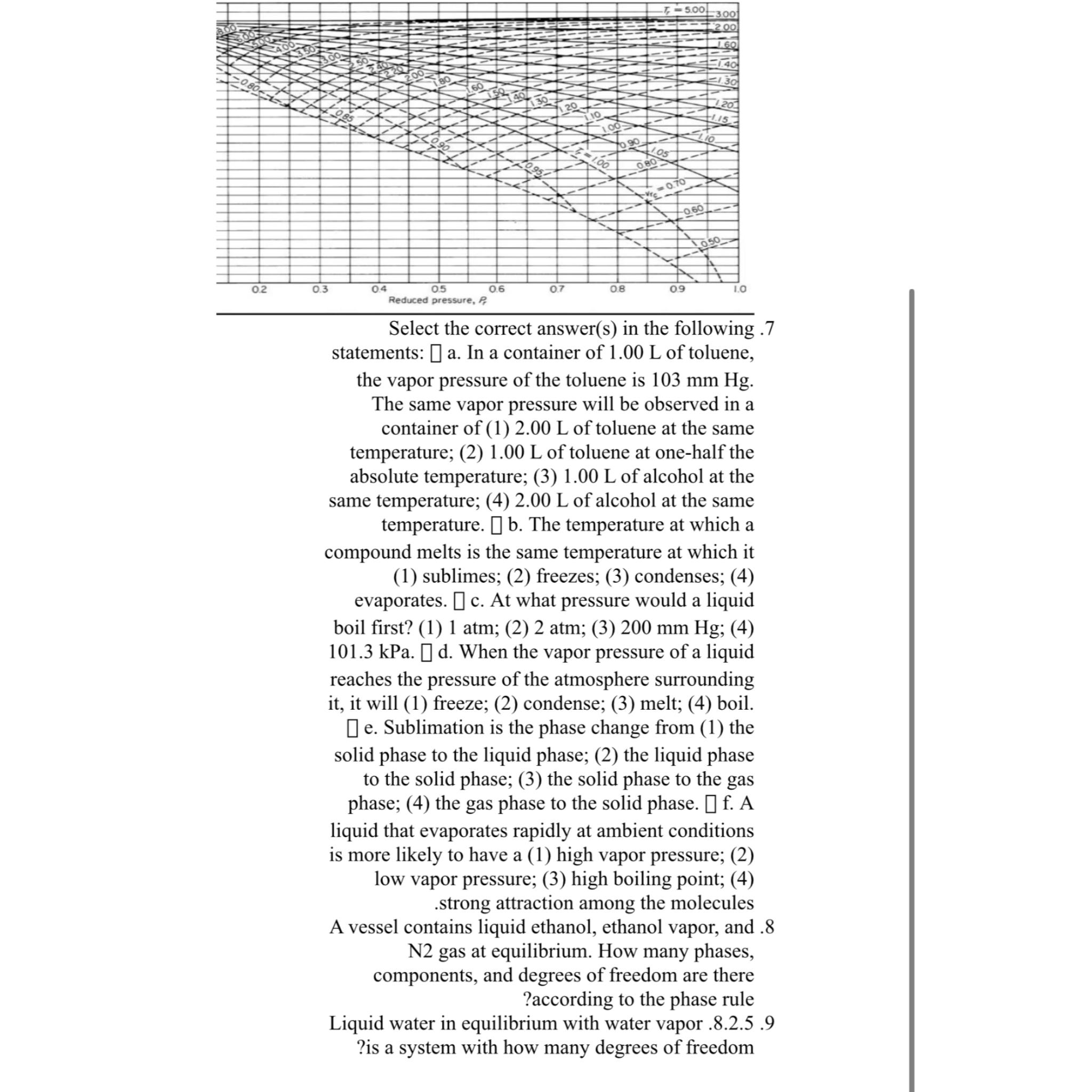
Select the correct answers in the following statements: a In a container of of toluene, the vapor pressure of the toluene is The same vapor pressure will be observed in a container of of toluene at the same temperature; of toluene at onehalf the absolute temperature; of alcohol at the same temperature; of alcohol at the same temperature. The temperature at which a compound melts is the same temperature at which it sublimes; freezes; condenses; evaporates. At what pressure would a liquid boil first? atm; atm; ; kPa.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


