Question
11 A gas with a mass of 3 kg has a volume of 3.33 m at a pressure of 135 kPa and temperature 40C.
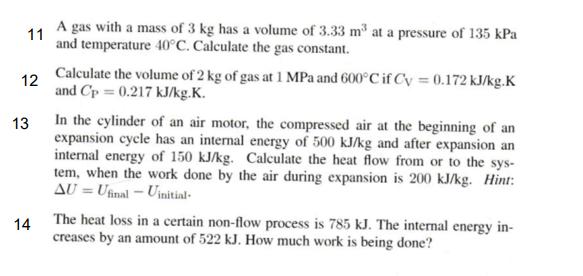
11 A gas with a mass of 3 kg has a volume of 3.33 m at a pressure of 135 kPa and temperature 40C. Calculate the gas constant. 12 13 14 Calculate the volume of 2 kg of gas at 1 MPa and 600C if Cy= 0.172 kJ/kg.K and Cp = 0.217 kJ/kg.K. In the cylinder of an air motor, the compressed air at the beginning of an expansion cycle has an internal energy of 500 kJ/kg and after expansion an internal energy of 150 kJ/kg. Calculate the heat flow from or to the sys- tem, when the work done by the air during expansion is 200 kJ/kg. Hint: AU = Ufinal - Uinitial- The heat loss in a certain non-flow process is 785 kJ. The internal energy in- creases by an amount of 522 kJ. How much work is being done?
Step by Step Solution
3.50 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
13 11 Given data Mass of gas m 3 kg Volume of gas V 333 m Pressure P 135 kPa ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Entrepreneurship Theory Process and Practice
Authors: Donald F. Kuratko
9th edition
1285531825, 1285051750, 9781285531823, 978-1285051758
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



