Question
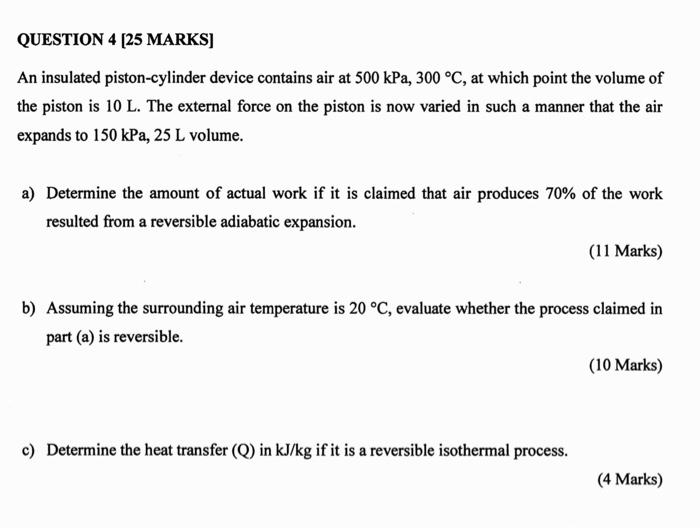
An insulated piston-cylinder device contains air at 500 kPa, 300 C, at which point the volume of the piston is 10 L. The external force
An insulated piston-cylinder device contains air at 500 kPa, 300 C, at which point the volume of the piston is 10 L. The external force on the piston is now varied in such a manner that the air expands to 150 kPa, 25 L volume.
a) Determine the amount of actual work if it is claimed that air produces 70% of the work resulted from a reversible adiabatic expansion.
b) Assuming the surrounding air temperature is 20 C, evaluate whether the process claimed in part (a) is reversible.
c) Determine the heat transfer (Q) in kJ/kg if it is a reversible isothermal process.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


