Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer a showing how its done on excel answer b and c also please answe asap and correctly 3) (30 marks] Standard aqueous solutions of
answer a showing how its done on excel
3) (30 marks] Standard aqueous solutions of FeCl2 are examined in a UV-Vis spectrometer. They yield the following absorbances at a given wavelength: Concentration, mg ml1 0 2 4 6 8 10 Absorbance 0.045 0.790 1.505 2.360 2.885 3.066 Blank sample number Absorbance 1 0.03 2 -0.02 3 -0.01 4 0.02 5 -0.02 6 0.03 -0.01 (a) Determine the unweighted least regression equation of the line and product-moment correlation coefficient, r, by processing the data in Excel. (b) Comment on the suitability of the linear regression fit to this data, providing evidence to support your opinion. (c) Assuming a linear correlation, calculate the concentration of a sample with an absorbance of 1.230 answer b and c also
please answe asap and correctly 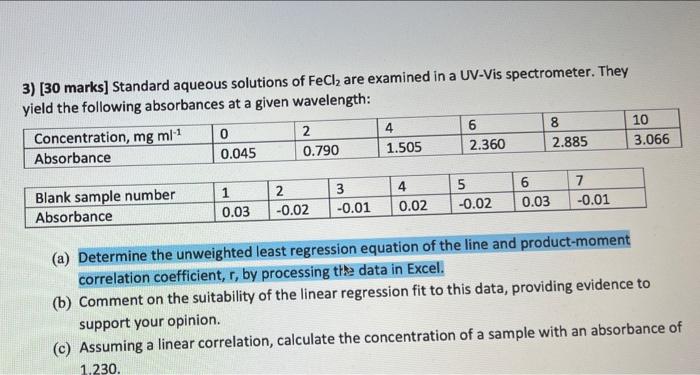

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


