Answer all of the 9 questions. I need all of them please!!! Ill thumb down for incompleted answers, up for completed. Answer all questions. Thanks
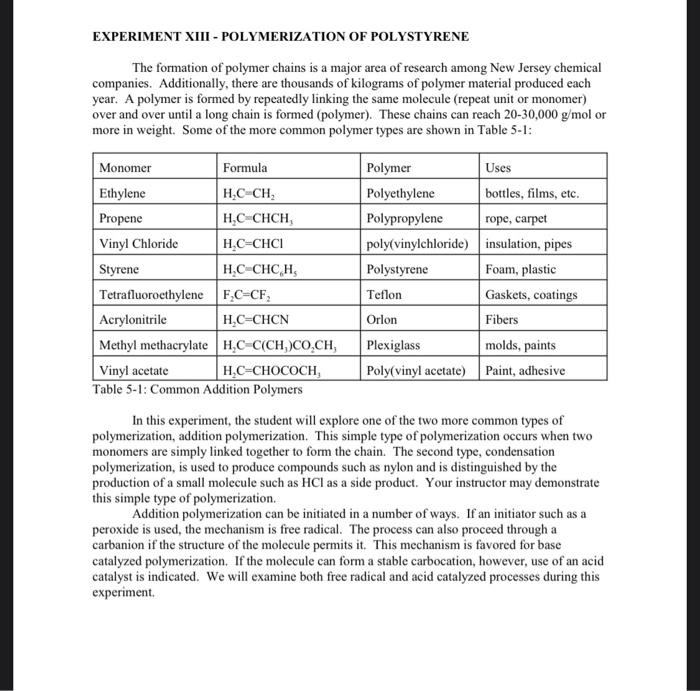
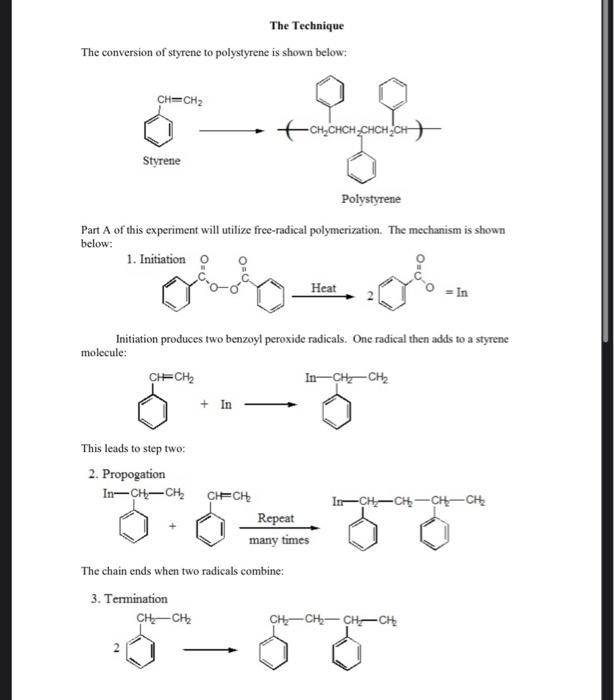
EXPERIMENT XIII - POLYMERIZATION OF POLYSTYRENE The formation of polymer chains is a major area of research among New Jersey chemical companies. Additionally, there are thousands of kilograms of polymer material produced each year. A polymer is formed by repeatedly linking the same molecule (repeat unit or monomer) over and over until a long chain is formed (polymer). These chains can reach 20-30,000 g/mol or more in weight. Some of the more common polymer types are shown in Table 5-1: Monomer Formula Polymer Uses Ethylene HC CH, Polyethylene bottles, films, etc. Propene H.C CHCH Polypropylene rope, carpet Vinyl Chloride H.C=CHCI poly(vinylchloride) insulation, pipes Styrene H.C CHCH. Polystyrene Foam, plastic Tetrafluoroethylene F.C=CF, Teflon Gaskets, coatings Acrylonitrile H,C=CHCN Orlon Fibers Methyl methacrylate H.C=C(CH)CO,CH, Plexiglass molds, paints Vinyl acetate H.C-CHOCOCH Poly(vinyl acetate) Paint, adhesive Table 5-1: Common Addition Polymers In this experiment, the student will explore one of the two more common types of polymerization, addition polymerization. This simple type of polymerization occurs when two monomers are simply linked together to form the chain. The second type, condensation polymerization, is used to produce compounds such as nylon and is distinguished by the production of a small molecule such as HCl as a side product. Your instructor may demonstrate this simple type of polymerization. Addition polymerization can be initiated in a number of ways. If an initiator such as a peroxide is used, the mechanism is free radical. The process can also proceed through a carbanion if the structure of the molecule permits it. This mechanism is favored for base catalyzed polymerization. If the molecule can form a stable carbocation, however, use of an acid catalyst is indicated. We will examine both free radical and acid catalyzed processes during this experiment The Technique The conversion of styrene to polystyrene is shown below: CH=CH2 -CH_CHCH_CHCHACHA Styrene Polystyrene Part A of this experiment will utilize free-radical polymerization. The mechanism is shown below: 1. Initiation o Heat In Initiation produces two benzoyl peroxide radicals. One radical then adds to a styrene molecule: CHECHE In-CH2 - CH2 + In This leads to step two: 2. Propogation In-CH-CH In-CH-CH-CH-CHE CHECHE Repeat many times The chain ends when two radicals combine: 3. Termination CHCH CH-CH2-CH-CH With the exception of the fact that you are making a polymer, this mechanism is the same as that for addition of HBr to an alkene and other standard addition reactions. Polystyrene can also be polymerized using an acid catalyst. The mechanism is thought to involve the formation of a carbocation. The mechanism is outlined below: A-C-CH CHECHE A-CHECH- Repeat many times -CH The base catalyzed process is essentially the same but proceeds through a carbanion. Required Chemicals and Non-Standard Equipment Part A Part B Prepared Styrene Prepared styrene benzoyl peroxide/toluene solution stannic chloride toluene toluene boiling chips methanol methanol vacuum filtration apparatus vacuum filtration apparatus microscope slides microscope slides 125 mL separatory funnel 125 mL separatory funnel 125 ml flask with cork Precautions STANNIC CHLORIDE IS HIGHLY TOXIC AND MUST BE USED IN THE HOOD! Do not leave the reflux unattended. Vent the separatory funnel. Don't breath any vapors from the organic solvents. Dispose of all organics as instructed Check the boiling flask for star-cracks and other hazards. Procedure Part A: Polymerization Using Benzoyl Peroxide 1. Obtain 5 mL of styrene and place it in a 50 mL round bottom flask. 2. Add 15 mL of benzoyl peroxide toluene solution to the styrene. 3. Add two boiling chips and set-up a standard reflux apparatus (see page 5). After your instructor has approved your set-up, reflux for 1 hour. While you are waiting, 5. After 1 hour, purify and isolate the polymer. First cool the flask to room temperature. 4. do part B. 6. 7. 8. 9. 1. 2. 3. 4. . 5. 6. 7. Pour the solution into a beaker containing 150 mL of methanol. Stir gently to coagulate the polymer Pour off the cloudy methanol and then add 25 mL of fresh methanol to the solid in the beaker. Isolate the solid by vacuum filtration. Weigh the polymer when it is dry. Cast a film as instructed in part C and attempt an IR. Part B: Polymerization Using an Acid Catalyst To a clean, dry 125 ml flask add 3 mL of styrene. IN THE HOOD, add 10 mL of toluene and 10 drops of stannic chloride. Stopper the flask and allow it to stand for 30 min. While one lab partner purifies the polystyrene from part A, the other uses the same procedure to purify the mixture from part B. IN THE HOOD, pour the polymer from the flask into a beaker containing 150 mL of methanol. Stir to coagulate the polymer. Pour off the cloudy methanol and then add 25 mL of fresh methanol to the solid. Isolate by vacuum filtration. Allow to dry and weigh. Cast the polymer as a film as instructed in part C and obtain an IR. Part C: Casting a Polymer Film Dissolve about 0.1 g of your polystyrene in 1-2 mL of toluene. Spread the mixture thinly on a microscope slide and allow to dry until next week in the hood. 3. After one week, remove a piece of the film with a razor blade and obtain an IR spectrum as demonstrated by your instructor. Data and Conclusions In this lab include: The appearance of the polymer through the various stages of the lab. The appearance of any solutions etc. The amount of polymer obtained in each procedure. The spectra from your films. Your polystyrene in a correctly labeled bottle. Supplementary Questions 1. The structure of methacrylate is shown below. Draw the structure of a methacrylate polymer that contains five repeat units. , 1. 2. 1. 2. 3. 4. H2C=c-c=0 CHE methyl methacrylate 2. Your instructor will give you a copy of the "C and 'H NMR of styrene. Assign each signal to the correct carbon or prolon. Questions 1. What would be a possible alternative initiation method in place of either benzoyl peroxide or an acid catalyst? 2. Why can't you use the styrene directly from the bottle? 3. Which of the two types of polymerization is being performed here? 4. What is the mechanism of the reaction? 5. What type of compounds can be used as drying agents? 6. When is a drying agent required? 7. What laboratory technique is used to perform the reaction? 8. Which spectroscopic method would be used to identify the product? 9. Draw the structure of polymethylmethacrylate using five repeat units. The structure of the methacrylate monomer can be found in the instructions