Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer all parts pls Chemistry Part A - Longhand Electron Configuration Use the patterns within the periodic table the following atoms 4. 321522s22p63s3p64s23d104p2 =k361s22s22p63s23p64s23d234p6 -
answer all parts pls 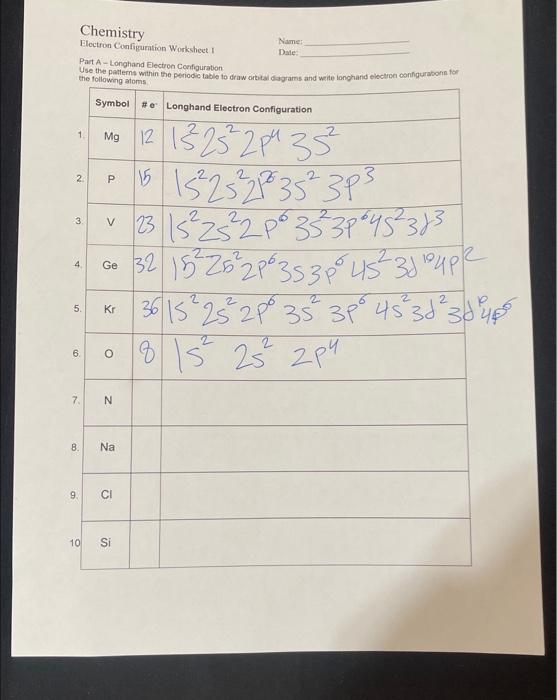
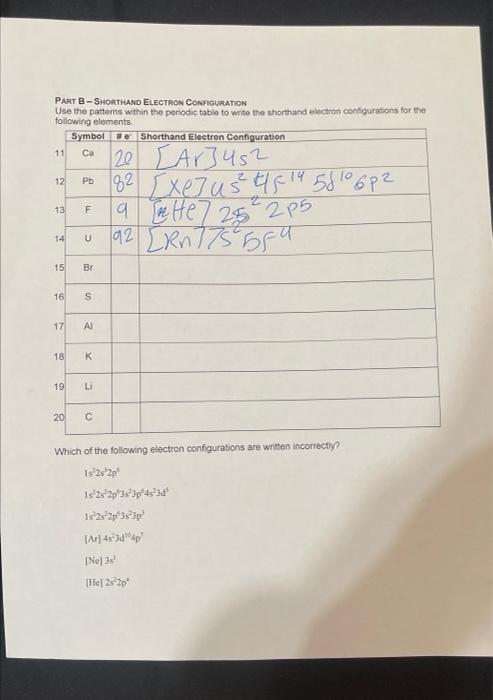
Chemistry Part A - Longhand Electron Configuration Use the patterns within the periodic table the following atoms 4. 321522s22p63s3p64s23d104p2 =k361s22s22p63s23p64s23d234p6 - 81s22s22p4 PART B - SHORTHAND ELECTRON CONFIGURATION Use the pattems within the periodic table to write the shorthand electron consgurations for the fnlliowing alemante Which of the following electron configurations are written incorrectly? Is2s32p6Is2s22p63s23p64s23s5Is2s22p13s23p3[As)4s23d64p3[Ne]3s1[He]2s22p4 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


