Answered step by step
Verified Expert Solution
Question
1 Approved Answer
in question number 5, it says net of the following equations above. The given kj/mol are the ones i solved which may not be right.
in question number 5, it says net of the following equations above. The given kj/mol are the ones i solved which may not be right. I am very confused on how am i supposed to be writing the net and enthalpy theory of it as a final answer 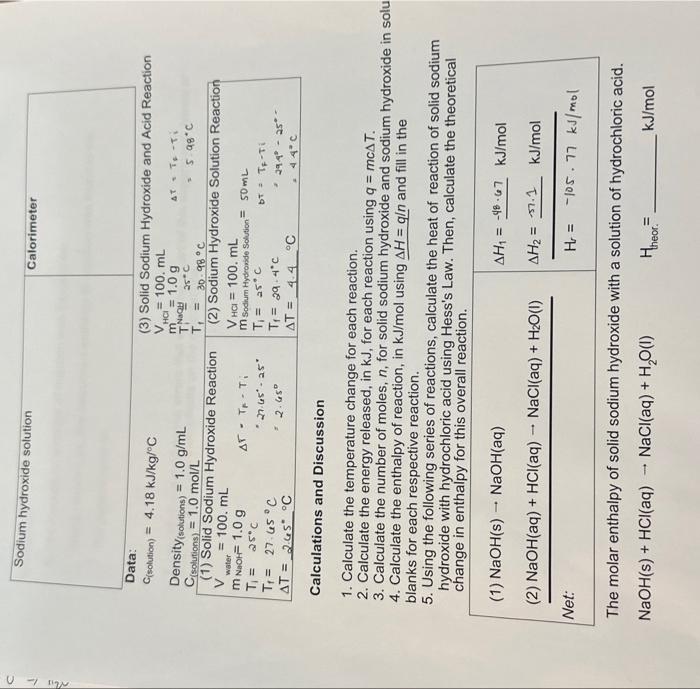
1. Calculate the temperature change for each reaction. 2. Calculate the energy released, in kJ, for each reaction using q=mcT. 3. Calculate the number of moles, n, for solid sodium hydroxide and sodium hydroxide in s 4. Calculate the enthalpy of reaction, in kJ/mol using H=q and fill in the blanks for each respective reaction. 5. Using the following series of reactions, calculate the heat of reaction of solid sodium hydroxide with hydrochloric acid using Hess's Law. Then, calculate the theoretical change in enthalpy for this overall reaction. The molar enthalpy of solid sodium hydroxide with a solution of hydrochloric acid. NaOH(s)+HCl(aq)NaCl(aq)+H2O(l)Htheor.= kJ/mol 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


