Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer all questions plz The pH of an aqueous solution is defined by the equation: pH=log[H+] Hence, the [H+]=10pH Find the [H+]if the pH=3.2. Report
answer all questions plz 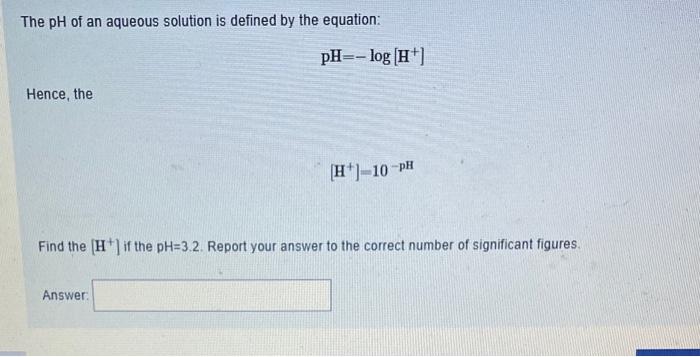
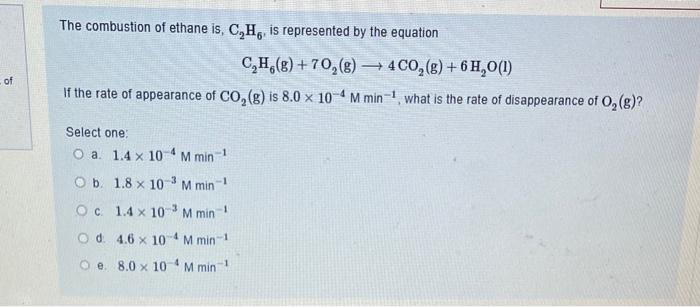

The pH of an aqueous solution is defined by the equation: pH=log[H+] Hence, the [H+]=10pH Find the [H+]if the pH=3.2. Report your answer to the correct number of significant figures. Answer: The combustion of ethane is, C2H6, is represented by the equation C2H6(g)+7O2(g)4CO2(g)+6H2O(l) If the rate of appearance of CO2(g) is 8.0104Mmin1, what is the rate of disappearance of O2(g) ? Select one: a. 1.4104Mmin1 b. 1.8103Mmin1 c. 1.4103Mmin1 d. 4.6104Mmin1 e. 8.0104Mmin1 A chemical reaction, A+B products, has a rate law of the form, rate=k[A]2[B]21. If the concentration of A is doubled and B is quadrupled (temperature constant) the rate of reaction increases by a factor of: Select one: a. 8 b. 4 c. 16 d. remains the same. e. 6 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


