Question
Choose the correct statement among the following. (A) Enthalpy of formation of a compound always have positive value. (B) Standard enthalpy of formation of
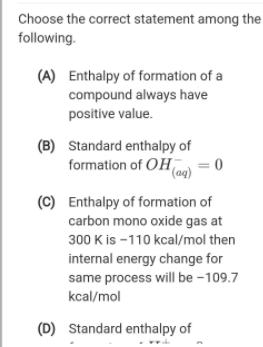
Choose the correct statement among the following. (A) Enthalpy of formation of a compound always have positive value. (B) Standard enthalpy of formation of OHmo = 0 (C) Enthalpy of formation of carbon mono oxide gas at 300 K is -110 kcal/mol then internal energy change for same process will be -109.7 kcal/mol (D) Standard enthalpy of
Step by Step Solution
3.50 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Statistics
Authors: Michael Sullivan III
4th Edition
978-032184460, 032183870X, 321844602, 9780321838704, 978-0321844606
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



