Question
Solve algebraically for the pH of a solution of 5 X 10^-3 moles of NaOCI in 1 L of water. Write the relevant equations
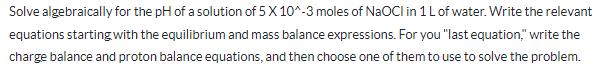
Solve algebraically for the pH of a solution of 5 X 10^-3 moles of NaOCI in 1 L of water. Write the relevant equations starting with the equilibrium and mass balance expressions. For you "last equation," write the charge balance and proton balance equations, and then choose one of them to use to solve the problem.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Marketing
Authors: Frederick Crane, Roger A. Kerin, Steven W. Hartley, William Rudelius
10th Canadian edition
1259268802, 978-1259268809
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



