Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Answer quickly for thumbs up Answer is CO+H2O(v)CO2+H2;Hr=41.15kJmol1at25C The product gas contains 40 mole% H2,40%CO2 and 20%H2O and leaves at a rate of 112molh1 and
Answer quickly for thumbs up 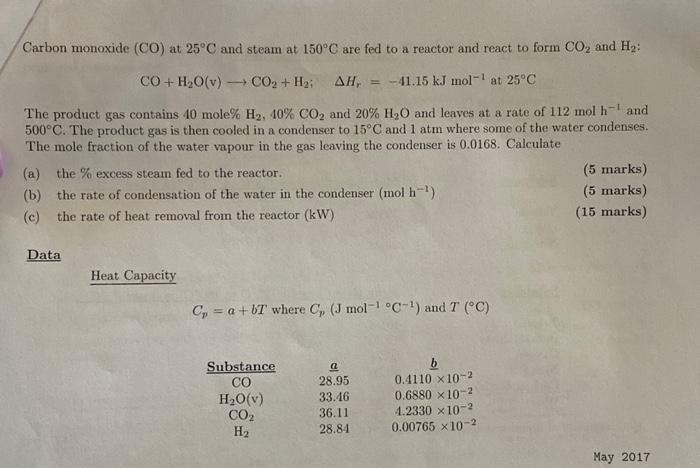
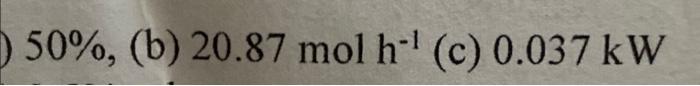
CO+H2O(v)CO2+H2;Hr=41.15kJmol1at25C The product gas contains 40 mole\% H2,40%CO2 and 20%H2O and leaves at a rate of 112molh1 and 500C. The product gas is then cooled in a condenser to 15C and 1 atm where some of the water condenses. The mole fraction of the water vapour in the gas leaving the condenser is 0.0168 . Calculate (a) the \% excess steam fed to the reactor. (5 marks) (b) the rate of condensation of the water in the condenser (molh1) (5 marks) (c) the rate of heat removal from the reactor ( kW) (15 marks) Data Heat Capacity Cp=a+bT where Cp(Jmol1C1) and T(C) 50%, (b) 20.87molh1 (c) 0.037kW 
Answer is
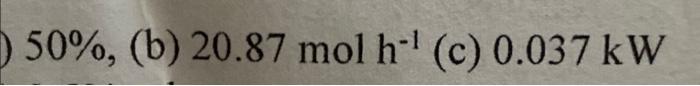
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


