Answered step by step
Verified Expert Solution
Question
1 Approved Answer
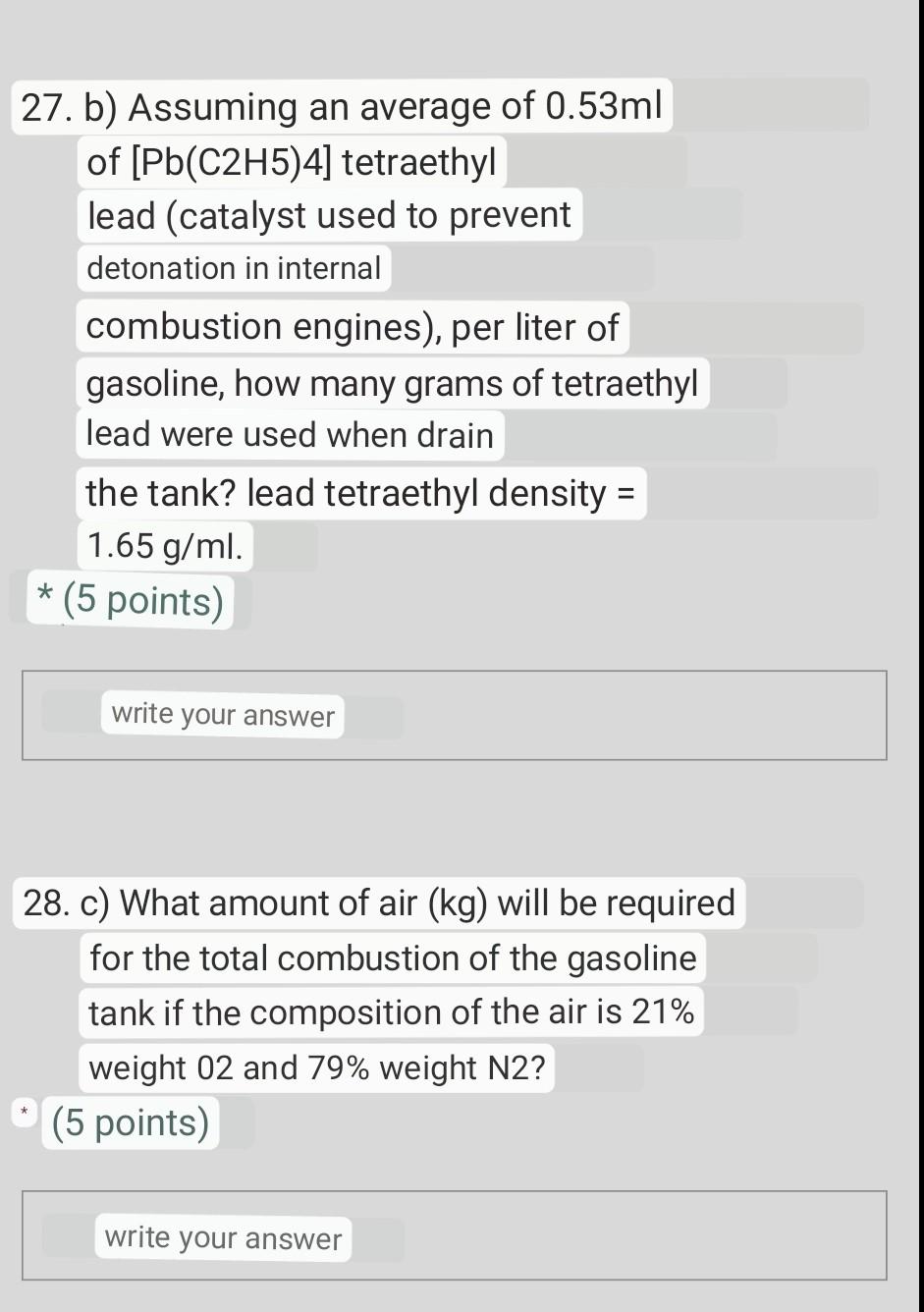
answer the 3 items 26. One of the ways to produce electricity is by using a gasoline engine like a car, connected to an electric

answer the 3 items
26. One of the ways to produce electricity is by using a gasoline engine like a car, connected to an electric generator. If an engine has a 35 liter tank which is loaded with premium gasoline (C8H18, density =0.97g/ml) and which has a fuel evaporative loss of 15% of its volume: a) Calculate the amount, in kg, of CO2 from the combustion of said fuel. The reaction that takes place is the following: 2C8H18+25O216CO2+18H2O *(5 points) 28. c) What amount of air (kg) will be required for the total combustion of the gasoline tank if the composition of the air is 21% weight 02 and 79% weight N2Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


