Use the information in Table 5G.2 to determine the value of K at 500 K for the
Question:
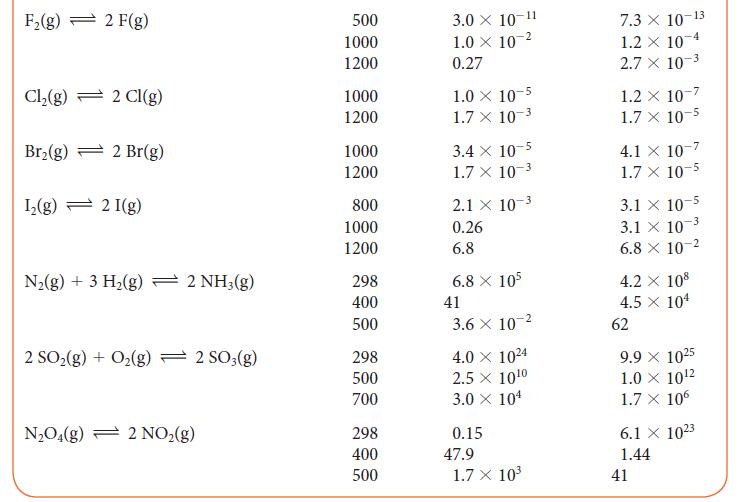
Use the information in Table 5G.2 to determine the value of K at 500 K for the reaction 2 NH3(g) + 3 I2(g) ⇌ N2(g) + 6 HI(g).
Transcribed Image Text:
TABLE 5G.2 Equilibrium Constants for Various Reactions Reaction H₂(g) + Cl₂(g) 2 HCl(g) H₂(g) + Br₂(g) → 2 HBr(g) H₂(g) + I₂(g) 2 HI(g) 2 BrCl(g) Br₂(g) + Cl₂(g) 2 HD(g) - H₂(g) + D₂(g) T/K* 300 500 1000 300 500 1000 298 500 700 300 500 1000 100 500 1000 K 4.0 × 10³1 4.0 × 10¹8 5.1 X 108 1.9 X 10¹7 1.3 × 10 ¹0 3.8 x 10¹ 794 160 54 377 32 5 0.52 0.28 0.26 K 4.0 × 10³1 4.0 × 10¹8 5.1 x 108 1.9 X 10¹7 1.3 × 10¹0 3.8 x 104 794 160 54 377 32 5 0.52 0.28 0.26
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
312g N2 6HI 2 NH31 Kc RTLog Ke AGAG AG products AG ran AG g products ...View the full answer

Answered By

Niala Orodi
I am a competent and an experienced writer with impeccable research and analytical skills. I am capable of producing quality content promptly. My core specialty includes health and medical sciences, but I can competently handle a vast majority of disciplines.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
(a) In an experiment, 5.0 mmol Cl 2 (g) was sealed into a reaction vessel of volume 2.0 L and heated to 1200. K, and the dissociation equilibrium was established. What is the equilibrium composition...
-
Use the information in Table 5G.2 to determine the value of K at 300 K for the reaction 2 BrCl (g) + H 2 (g) Br 2 (g) + 2 HCl(g). TABLE 5G.2 Equilibrium Constants for Various Reactions Reaction H(g)...
-
Use the information in Table 2.5 to predict the standard reaction enthalpy of2 H2 (g) + 02(g) 2 H2O (1) at 100C from its value at 25C.
-
Apply Theorem 3 to calculate the matrix exponential e At for each of the matrices in Problems 35 through 40. A = 13 3 3 3 01 3 3 00 23 0002
-
Five hundred kmol/h of a liquid mixture of light alcohols containing, by moles, 40% methanol (M), 35% ethanol (E), 15% isopropanol (IP), and 10% normal propanol (NP) is distilled in a sequence of two...
-
If 60 C of charge pass through an electric conductor in 30 seconds, determine the current in the conductor.
-
(c) Hence obtain the shrunk mean value of log(house price) for each town.
-
Quigley Inc. is considering two financial plans for the coming year. Management expects sales to be $301,770, operating costs to be $266,545, assets to be $200,000, and its tax rate to be 35%. Under...
-
Boulder Furniture has bonds outstanding that mature in 15 years, have a 6 percent coupon, and pay interest annually. These bonds have a face value of $1,000 and a current market price of $1,075. What...
-
Gibson Agency Case: 1. Calculate and present the budgeted profit for each of Gibson's clients for each of the years 2016 through 2019, using the current costing system (i.e., the one described in the...
-
Two unknown molecular compounds were being studied. A solution containing 5.00 g of compound A in 100. g of water froze at a lower temperature than a solution containing 5.00 g of compound B in 100....
-
One step in the manufacture of sulfuric acid is the formation of sulfur trioxide by the combustion of SO 2 with O 2 in the presence of a vanadium(V) oxide catalyst. Suppose you are working out how to...
-
Test each of the following differentials for exactness. (a) du = xy dx + xy dy, (b) du = y e axy dx + x e axy dy.
-
In addition to the strongest military in the world, the United States wields enormous soft power. Define soft power. What factors make the United States powerful when it comes to soft power?
-
Tampa by the Bay Cardiology practice is experiencing long wait times for new patient appointments. Next available appointment is 30 days. The administrator has asked the practice manager to construct...
-
In 2013, Idalia Hernndez Ramos, a middle school teacher in Mexico, was a victim of cyber harassment. After discovering that one of her students tweeted that the teacher was a "bitch" and a "whore,"...
-
Your life couldn't be any better. You just accepted a new role as a senior consultant for a project management services firm in San Francisco, and the move is finally happening. You've got a great...
-
What are the two "engines" that drive earth's processes, how do they work (basically) and what are their energy sources? How do the "engines" influence and interact with the Earth Systems? (provide a...
-
a) Why is speed of response important? b) Why is accuracy of response important? c) Define incident response in terms of planning. d) Why are rehearsals important? e) What is a walkthrough or...
-
Based on the scenario described below, generate all possible association rules with values for confidence, support (for dependent), and lift. Submit your solutions in a Word document (name it...
-
Propose a mechanism for the following transformation: 1) Excess LAH 2) H20 CH3
-
A carbocation is resonance stabilized when it is adjacent to an oxygen atom: Such a carbocation is even more stable than a tertiary carbocation. Using this information, propose a mechanism for the...
-
One liter of fully oxygenated blood can carry 0.18 liters of O 2 measured at T = 298 K and P = 1.00 atm. Calculate the number of moles of O 2 carried per liter of blood. Hemoglobin, the oxygen...
-
4 Exercise 9-6 (Algo) Lower of cost or market [LO9-1) 75 Tatum Company has four products in its inventory. Information about the December 31, 2021, Inventory is as follows: oints Product Total Cost...
-
A real estate investment is expected to return to its owner $3,500 per year for 16 years after expenses. At the end of year 16, the property is expected to be sold for $49,000. Assuming the required...
-
You borrowed $15,000 for buying a new car from a bank at an interest rate of 12% compounded monthly. This loan will be repaid in 48 equal monthly installments over four years. Immediately after the...

Study smarter with the SolutionInn App


