Answered step by step
Verified Expert Solution
Question
1 Approved Answer
any x=4 Q3 The following are the experimental results of VLE of isopropanol (1) - water (2) mixture carried out at American Chemical Society lab
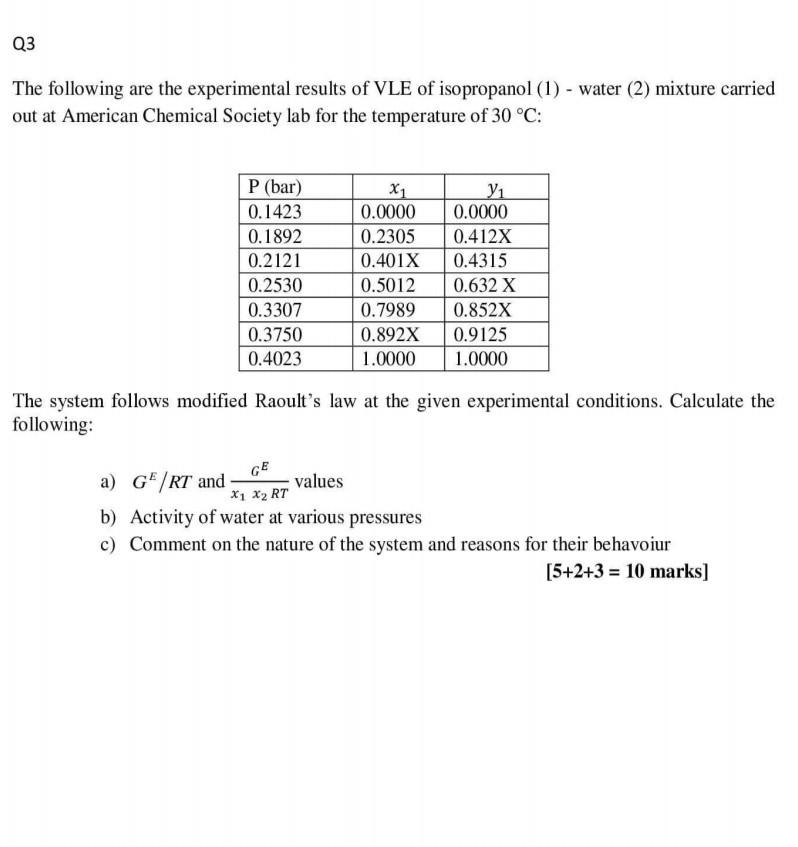
any x=4
Q3 The following are the experimental results of VLE of isopropanol (1) - water (2) mixture carried out at American Chemical Society lab for the temperature of 30 C: P (bar) 0.1423 0.1892 0.2121 0.2530 0.3307 0.3750 0.4023 X1 0.0000 0.2305 0.401X 0.5012 0.7989 0.892X 1.0000 1 0.0000 0.412X 0.4315 0.632 x 0.852X 0.9125 1.0000 The system follows modified Raoult's law at the given experimental conditions. Calculate the following: GE a) GF/RT and values X1 X2 RT b) Activity of water at various pressures c) Comment on the nature of the system and reasons for their behavoiur [5+2+3 = 10 marks)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


