Answered step by step
Verified Expert Solution
Question
1 Approved Answer
applied chemical principles 1. Estimate the solubility of calcium curbonate at 25C in the presence of 0.09MCaCl (aq). [7] 2. Will BaSO4 precipitate from a
applied chemical principles 
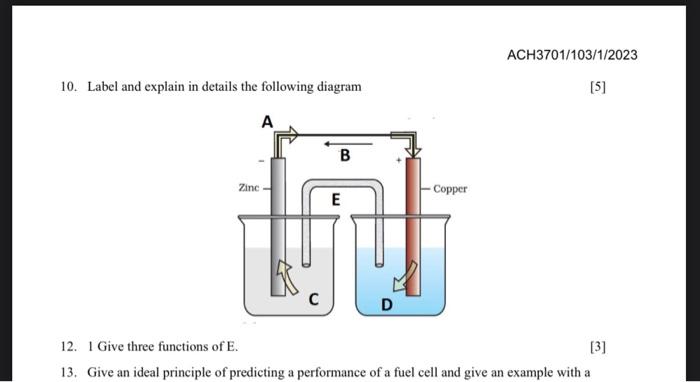
1. Estimate the solubility of calcium curbonate at 25C in the presence of 0.09MCaCl (aq). [7] 2. Will BaSO4 precipitate from a solution containing 1.5104MBa+2, barium ion, if enough soluble salt, Na2SO4, is added to make the solution 2.5104M in SO42? 3. Describe oxidation reaction and use a rust of iron as an example. [5] 4. Describe the formation of a char coal. Motivate your answer with chemical reactions. [5] 5. List three possible disadvantages when a car owner replaces the manufacturer's recommended gasoline with methanol. [3] 6. Consider a galvanic cell consisting of 5Fe2+(aq)+MnO4(aq)+8H+(aq)5Fe3(aq)+Mn2+(aq)+4H2O(h) i) Write the oxidation and reduction half-reactions ii) Write the reaction using cell notation. iii) Which reaction occurs at the cathode? iv) Which reaction occurs at the anode? [6] 7. The solubility product of calcium fluoride (CaF2) is 3.451011. If 1.5mL of a 0.10M solution of NaF is added to 130mL of a 1.0105M solution of Ca(NO3)2, will CaF2 precipitate? [5] 8. Represent the cell in which the following reaction takes place Mg(s)+2Ag+(0.001M)Mg2+(0.130M)+2Ag(s) Calculate its Eimit if Eicatio=3.17v 9. The resistance of a conductivity cell containing 0.001MKCl solution at 298K is 1500. What is the cell constant if conductivity of 0.001MKCl solution at 298K is 0.146103Scm !. 10. Label and explain in details the following diagram [5] 12. 1 Give three functions of E. [3] 13. Give an ideal principle of predicting a performance of a fuel cell and give an example with a 1. Estimate the solubility of calcium curbonate at 25C in the presence of 0.09MCaCl (aq). [7] 2. Will BaSO4 precipitate from a solution containing 1.5104MBa+2, barium ion, if enough soluble salt, Na2SO4, is added to make the solution 2.5104M in SO42? 3. Describe oxidation reaction and use a rust of iron as an example. [5] 4. Describe the formation of a char coal. Motivate your answer with chemical reactions. [5] 5. List three possible disadvantages when a car owner replaces the manufacturer's recommended gasoline with methanol. [3] 6. Consider a galvanic cell consisting of 5Fe2+(aq)+MnO4(aq)+8H+(aq)5Fe3(aq)+Mn2+(aq)+4H2O(h) i) Write the oxidation and reduction half-reactions ii) Write the reaction using cell notation. iii) Which reaction occurs at the cathode? iv) Which reaction occurs at the anode? [6] 7. The solubility product of calcium fluoride (CaF2) is 3.451011. If 1.5mL of a 0.10M solution of NaF is added to 130mL of a 1.0105M solution of Ca(NO3)2, will CaF2 precipitate? [5] 8. Represent the cell in which the following reaction takes place Mg(s)+2Ag+(0.001M)Mg2+(0.130M)+2Ag(s) Calculate its Eimit if Eicatio=3.17v 9. The resistance of a conductivity cell containing 0.001MKCl solution at 298K is 1500. What is the cell constant if conductivity of 0.001MKCl solution at 298K is 0.146103Scm !. 10. Label and explain in details the following diagram [5] 12. 1 Give three functions of E. [3] 13. Give an ideal principle of predicting a performance of a fuel cell and give an example with a 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


