Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Apply the steady-state approximation to the following mechanism, in which X and Z are final products and X is an unstable intermedinte: k1 A+ B
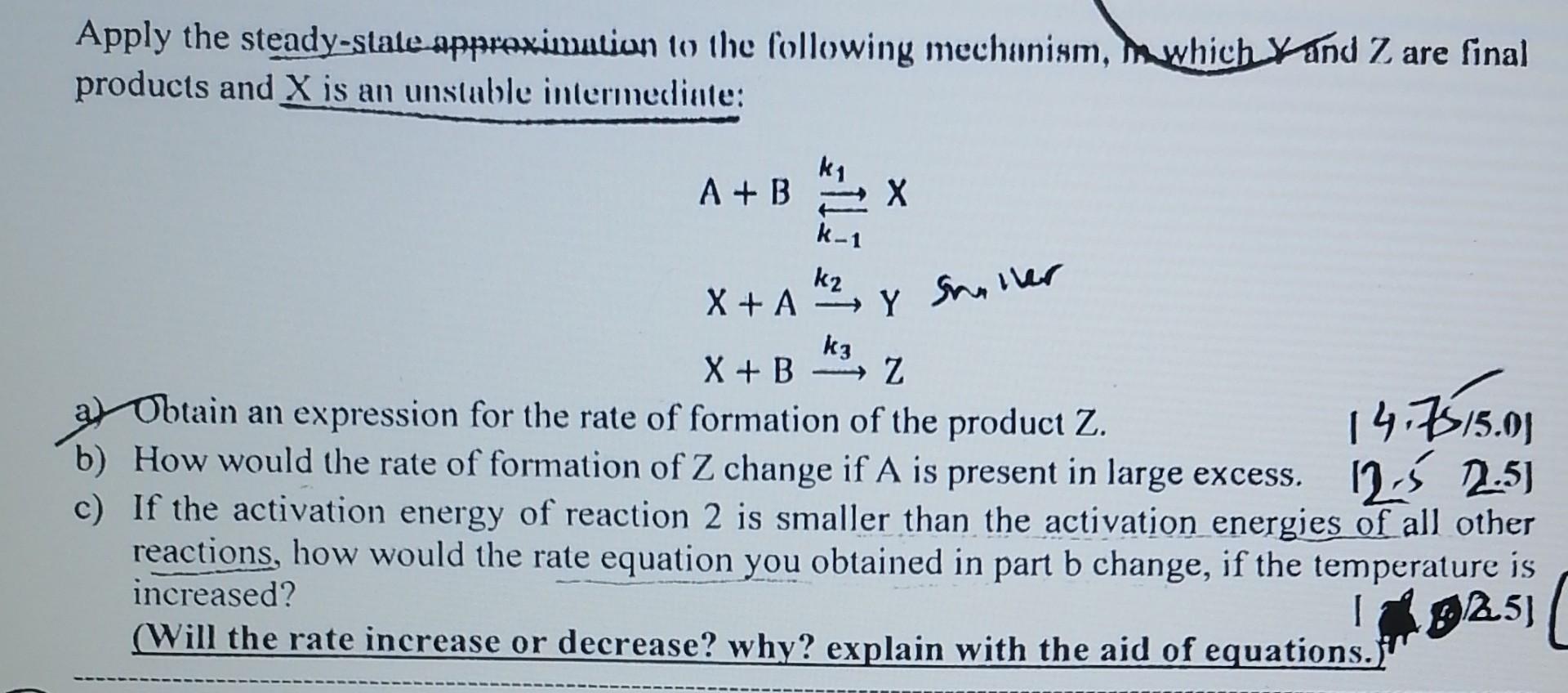
Apply the steady-state approximation to the following mechanism, in which X and Z are final products and X is an unstable intermedinte: k1 A+ B 2 X A K-1 X+A Y Siler Y k2 X + A k3 X + B z a) Obtain an expression for the rate of formation of the product Z. 14.7515.03 b) How would the rate of formation of Z change if A is present in large excess. 12-5 2.5) c) If the activation energy of reaction 2 is smaller than the activation energies of all other reactions, how would the rate equation you obtained in part b change, if the temperature is increased? 1 (Will the rate increase or decrease? why? explain with the aid of equations. 925)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


