Question
The passivation potential of iron is a function of pH. At one time it was proposed to identify iron's passive film by calculating the
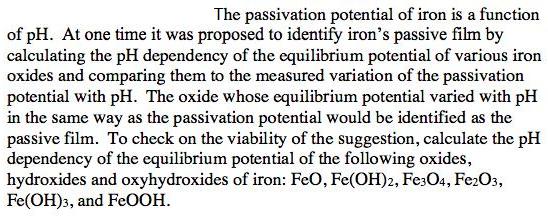
The passivation potential of iron is a function of pH. At one time it was proposed to identify iron's passive film by calculating the pH dependency of the equilibrium potential of various iron oxides and comparing them to the measured variation of the passivation potential with pH. The oxide whose equilibrium potential varied with pH in the same way as the passivation potential would be identified as the passive film. To check on the viability of the suggestion, calculate the pH dependency of the equilibrium potential of the following oxides, hydroxides and oxyhydroxides of iron: FeO, Fe(OH)2, Fe:O4, Fe2O3, Fe(OH)3, and FeOOH.
Step by Step Solution
3.45 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managerial Economics Markets and the Firm
Authors: William Boyes
2nd edition
618988629, 978-0618988624
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



