Answered step by step
Verified Expert Solution
Question
1 Approved Answer
One hundred grams of iron oxide (FeO) is reacted with 1000 cm 3 of pure hydrogen(STP) in a closed system at one atmosphere using a
One hundred grams of iron oxide (FeO) is reacted with 1000 cm3 of pure hydrogen(STP) in a closed system at one atmosphere using a temperature of 400 oC for an extended time. What is the weight of the pure Fe that would be formed? Assume that FeO is stable at 400 oC (WH2 = 2 gm/mol; WFe =56 gm/mole)
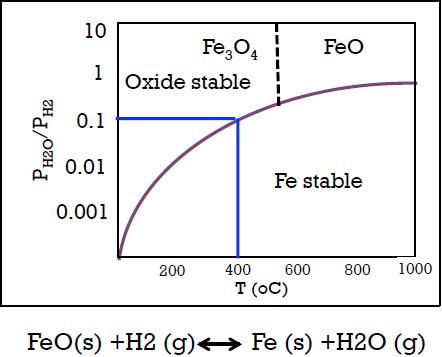
10 Fe,04 Feo 1 Oxide stable 0.1 0.01 Fe stable 0.001 200 400 600 800 1000 T (OC) FeO(s) +H2 (g) Fe (s) +H2O (g) PH20/PH2
Step by Step Solution
★★★★★
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


