Answered step by step
Verified Expert Solution
Question
1 Approved Answer
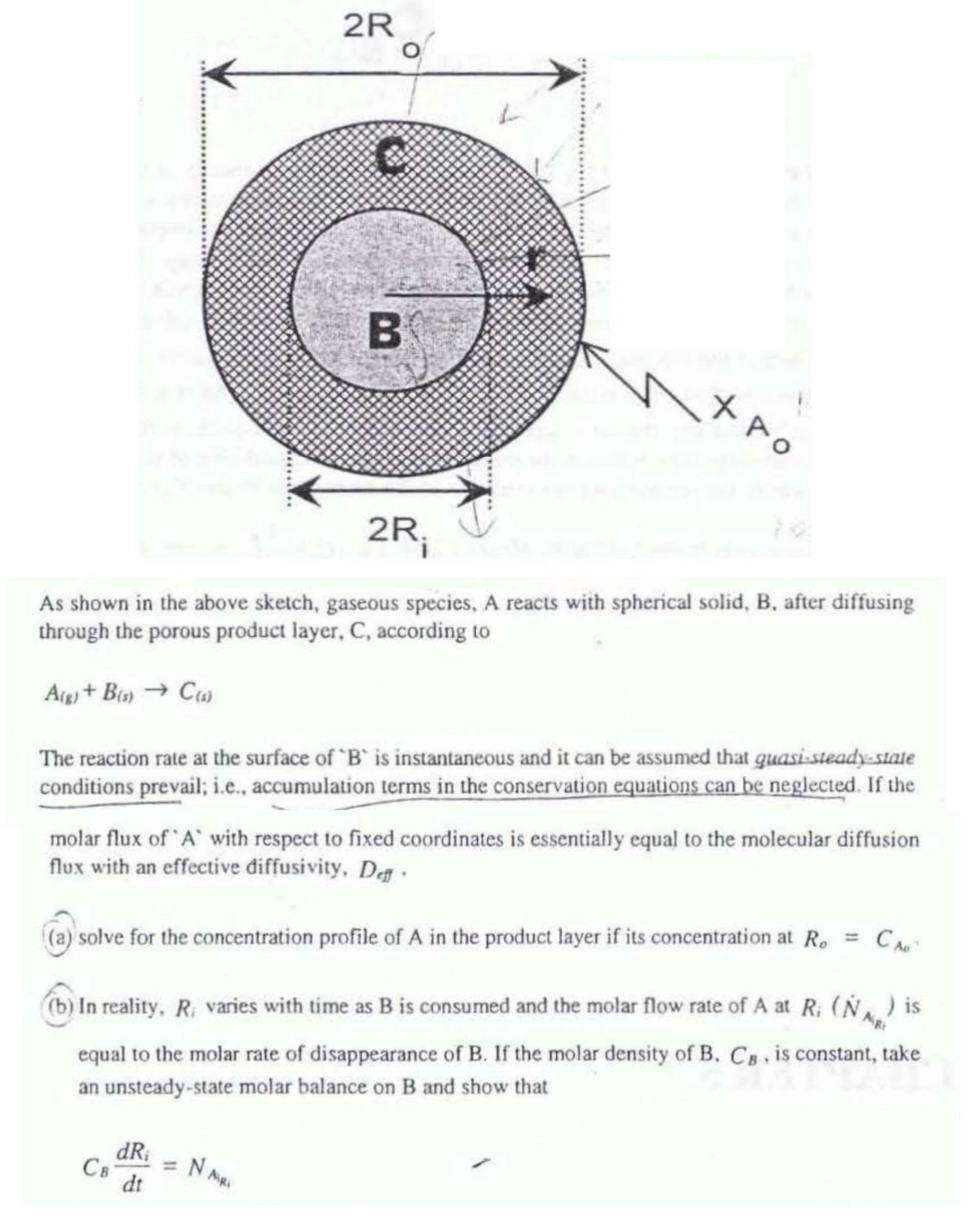
As shown in the above sketch, gaseous species, A reacts with spherical solid, B , after diffusing through the porous product layer, C , according
As shown in the above sketch, gaseous species, A reacts with spherical solid, B after diffusing through the porous product layer, according to
The reaction rate at the surface of is instantaneous and it can be assumed that quasisteadystate conditions prevail; ie accumulation terms in the conservation equations can be neglected. If the molar flux of with respect to fixed coordinates is essentially equal to the molecular diffusion flux with an effective diffusivity,
a solve for the concentration profile of in the product layer if its concentration at
b In reality, varies with time as is consumed and the molar flow rate of at is equal to the molar rate of disappearance of If the molar density of is constant, take an unsteadystate molar balance on and show that
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


