Answered step by step
Verified Expert Solution
Question
1 Approved Answer
ASAP A rigid container contains 0.15kg of saturated vapour at 2.4 bar. The container is cooled and the pressure drops to 1.2. Calculate the heat
ASAP 
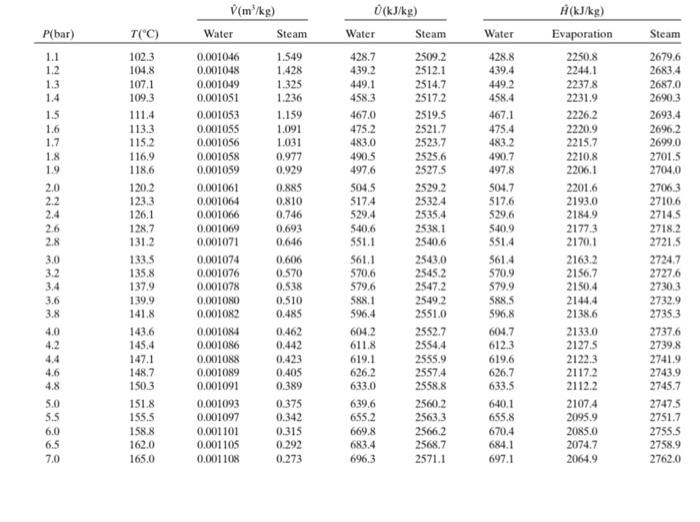
A rigid container contains 0.15kg of saturated vapour at 2.4 bar. The container is cooled and the pressure drops to 1.2. Calculate the heat transfer to/from the container, in KJ. Neglect kinetic and potential energy changes. Note that the reported heat transfer must be negative is energy is leaving the container and positive if it enters it. Your Answer: Answer Hide hint for Question 3 - It is closed system - As the container is rigid, the volume and total mass will stay the same between the initial and final state. - This means that the specific volume of water will stay constant. - Use the information in the bullet above to fine the quality of the mixture at the final state. Then, use the quality to perform the energy balance 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


