Assume that Torrey Nanos drug candidate is successful in Phase I and II- its first product to make it to human trials, let alone manufacturing.
Assume that Torrey Nano’s drug candidate is successful in Phase I and II- its first product to make it to human trials, let alone manufacturing. It now needs to manufacture a large amount of the drug for Phase III testing.
Question: Should they vertically integrate into manufacturing?
- Apply concepts from both the transaction cost view and the capabilities view to support your argument (and if the views don’t both support your recommendation, why did you choose the direction you did).

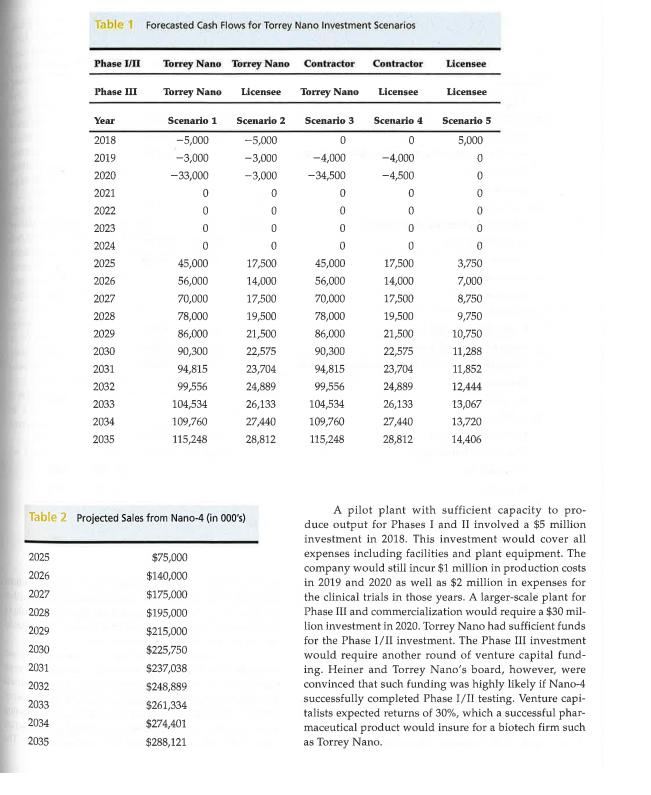


Jack Heiner, the CEO of Torrey Nano, a seven-year-old bio- technology firm, faced an important decision in the spring of 2017. Torrey Nano, based in San Diego, California was a company of 35 employees, most of whom were research sci- entists. Torrey Nano's pharmaceutical product, codename Nano-4, was almost ready for human testing. The company aspired to be a full-fledged pharmaceutical company. How- ever, like most biotech startups, it had focused on R&D since its founding. Consequently, it had done no manufacturing or marketing. With the impending human testing of Nano-4, however, it faced the decision of whether to build a small- scale pilot plant to manufacture Nano-4 for human trials. Nano-4 was the fourth product that Torrey Nano had devel- oped but the first to advance to human trials. The drug had multiple potential therapeutic applications, but Torrey Nano had focused on diabetes treatment as its first application. The company had three other products in earlier stages of development. Human testing involved three phases. Phase I, which lasted from 6-12 months, assessed basic human safety (e.g., adverse side effects, etc.). If a product was approved in Phase I, it moved to Phase II where the drug was tested with a small group of patients. Phase II examined the efficacy of a drug in treating a disease and also evaluated whether serious side effects occurred. Phase II usually lasted from one to two years. Products that succeeded in Phases I and II could go on to Phase III. Phase III, which could take two to five years to complete, tested the drug using a large sample of patients. Regulations required that whatever manufac- turing processes were used in Phase III must be the same as those used after the drug was approved and marketed commercially. The manufacturing of drugs based on nanotechnol- ogy was considered highly complex and uncertain. Drugs based on nanotechnology were particularly new in 2017 but were generally thought to hold great promise for a wide variety of diseases. Several reports suggested that manu- facturing nano-materials was both difficult to replicate and scale. Thus, it was challenging to develop effective processes for manufacturing new nano-drugs. Some nano- technology drugs, while feasible to make in the small quan- tities needed for pilot studies, were especially problematic to manufacture on a large scale. Very small differences in * This case is intended for class discussion only. Torrey Nano is a fictitious company and, while nanotechnology is believed to offer great promise for the future in pharmaceuticals, technical details in the case may not reflect reality. processes could lead to failure. Moreover, industry experts reported that firms often had difficulty transferring a manu facturing process from one facility to another. Few young companies had experience manufacturing products of the type of Nano-4. There were, however, a handful of contract manufacturers who had some experience in manufacturing involving nanotechnology. Even for these firms, though, often required considerable trial and error before mastering the process for a particular drug. Torrey Nano managers identified five possible scenar- ios for how they might handle the manufacturing of Nano- The five scenarios are listed here with cash flow estimates included in Table 1. Scenario 1: Torrey Nano builds a pilot plant for Phases I/II and a full-scale plant for Phase III. Scenario 2: Torrey Nano builds a pilot plant for Phases I/II and licenses out the manufacturing rights for Phase III. Scenario 3: Contract out manufacturing for Phases 1/ II, build a full-scale plant in-house for Phase III. Scenario 4: Contract out manufacturing for Phases 1/ II and license out manufacturing rights for Phase III. Scenario 5: Manufacturing in all phases would be done by licensee. In all five scenarios, Nano-4 would ultimately be dis tributed and marketed by an established pharmaceutical firm that would serve as Torrey Nano's and/or the eventual manufacturer's, marketing partner. If Nano-4 successfully navigated clinical testing to reach the commercial stage, ana- lysts projected sales ranging from $75 million in 2025 to over $288 million in 2035, the last year of patent protection (see Table 2). As Heiner contemplated his options, it was clear that the scenarios involving in-house manufacturing were costlier, but potentially more lucrative. Both of the first two scenarios required capital investments in 2018 and 2019 to build a pilot plant while scenarios 1 and 3 required large capital outlays to build a full-scale plant in 2020 and 2021 (see Table 3). Cash flow projections suggested that build ing a full-scale plant would allow Torrey Nano to capture a higher percentage of the revenue stream for Nano-4-$15 million licensing fee from its marketing partner in 2025 upon successful completion of clinical trials and 40 percent of royalties. Licensing the manufacturing rights after Phase II, as required by Scenarios 2 and 4, would yield a lower payout $10 million licensing fee from the marketing part ner and 10 percent royalties.
Step by Step Solution
3.57 Rating (171 Votes )
There are 3 Steps involved in it
Step: 1
Question Yes they should vertically merge manufacturing This is because Torrey Nanos drug is the fir...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


