
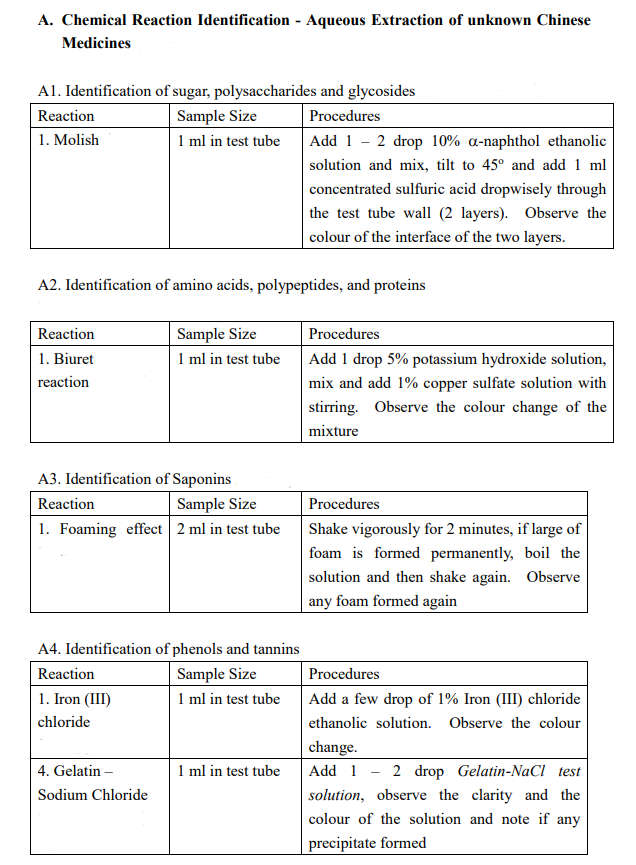
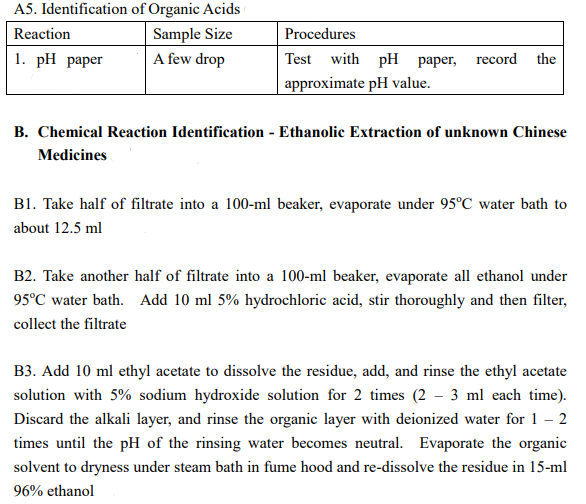
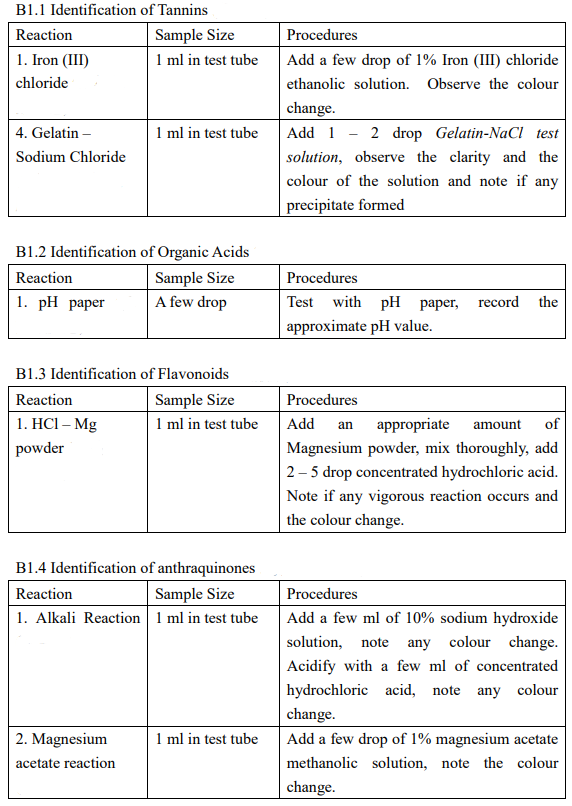
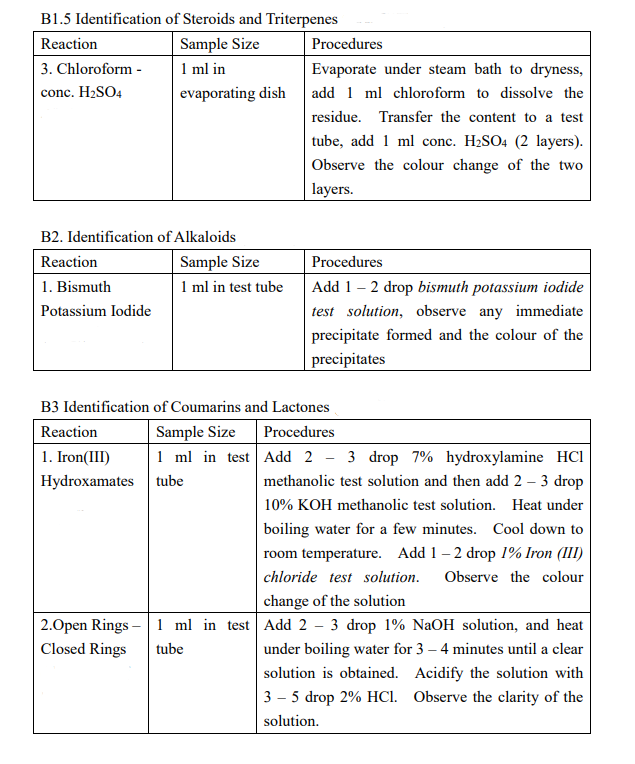
(Astragalus membranaceus (Fisch.) Bung) Description of sample species Data / Expected result including color change Aqueous extraction : A1-A5 Ethanolic extraction : B1.1-B1.5; B2, B3.1-B3.2 A. Chemical Reaction Identification - Aqueous Extraction of unknown Chinese Medicines A1. Identification of sugar, polysaccharides and glycosides Reaction Sample Size Procedures 1. Molish 1 ml in test tube Add 1 - 2 drop 10% a-naphthol ethanolic solution and mix, tilt to 45 and add 1 ml concentrated sulfuric acid dropwisely through the test tube wall (2 layers). Observe the colour of the interface of the two layers. A2. Identification of amino acids, polypeptides, and proteins Reaction 1. Biuret reaction Sample Size 1 ml in test tube Procedures Add 1 drop 5% potassium hydroxide solution, mix and add 1% copper sulfate solution with stirring. Observe the colour change of the mixture A3. Identification of Saponins Reaction Sample Size 1. Foaming effect 2 ml in test tube Procedures Shake vigorously for 2 minutes, if large of foam is formed permanently, boil the solution and then shake again. Observe any foam formed again A4. Identification of phenols and tannins Reaction Sample Size Procedures 1. Iron (III) 1 ml in test tube Add a few drop of 1% Iron (III) chloride chloride ethanolic solution. Observe the colour change. 4. Gelatin 1 ml in test tube Add 1 - 2 drop Gelatin-NaCl test Sodium Chloride solution, observe the clarity and the colour of the solution and note if any precipitate formed A5. Identification of Organic Acids Reaction Sample Size 1. pH paper A few drop Procedures Test with pH paper, record the approximate pH value. B. Chemical Reaction Identification - Ethanolic Extraction of unknown Chinese Medicines B1. Take half of filtrate into a 100-ml beaker, evaporate under 95C water bath to about 12.5 ml B2. Take another half of filtrate into a 100-ml beaker, evaporate all ethanol under 95C water bath. Add 10 ml 5% hydrochloric acid, stir thoroughly and then filter, collect the filtrate B3. Add 10 ml ethyl acetate to dissolve the residue, add, and rinse the ethyl acetate solution with 5% sodium hydroxide solution for 2 times (2 3 ml each time). Discard the alkali layer, and rinse the organic layer with deionized water for 1 2 times until the pH of the rinsing water becomes neutral. Evaporate the organic solvent to dryness under steam bath in fume hood and re-dissolve the residue in 15-ml 96% ethanol B1.1 Identification of Tannins Reaction Sample Size 1. Iron (III) 1 ml in test tube chloride Procedures Add a few drop of 1% Iron (III) chloride ethanolic solution. Observe the colour change. Add 1 - 2 drop Gelatin-NaCl test solution, observe the clarity and the colour of the solution and note if any precipitate formed 1 ml in test tube 4. Gelatin - Sodium Chloride B1.2 Identification of Organic Acids Reaction Sample Size 1. pH paper A few drop Procedures Test with pH paper, approximate pH value. record the B1.3 Identification of Flavonoids Reaction Sample Size 1. HCI - Mg 1 ml in test tube powder amount Procedures Add an appropriate of Magnesium powder, mix thoroughly, add 2 - 5 drop concentrated hydrochloric acid. Note if any vigorous reaction occurs and the colour change. B1.4 Identification of anthraquinones Reaction Sample Size 1. Alkali Reaction 1 ml in test tube Procedures Add a few ml of 10% sodium hydroxide solution, note any colour change. Acidify with a few ml of concentrated hydrochloric acid, note any colour change. Add a few drop of 1% magnesium acetate methanolic solution, note the colour change. 1 ml in test tube 2. Magnesium acetate reaction B1.5 Identification of Steroids and Triterpenes Reaction Sample Size Procedures 3. Chloroform - 1 ml in Evaporate under steam bath to dryness, conc. H2SO4 evaporating dish add 1 ml chloroform to dissolve the residue. Transfer the content to a test tube, add 1 ml conc. H2SO4 (2 layers). Observe the colour change of the two layers. B2. Identification of Alkaloids Reaction Sample Size 1. Bismuth 1 ml in test tube Potassium Iodide Procedures Add 1 - 2 drop bismuth potassium iodide test solution, observe any immediate precipitate formed and the colour of the precipitates B3 Identification of Coumarins and Lactones Reaction Sample Size Procedures 1. Iron(III) 1 ml in test Add 2 - 3 drop7% hydroxylamine HCI Hydroxamates tube methanolic test solution and then add 2 - 3 drop 10% KOH methanolic test solution. Heat under boiling water for a few minutes. Cool down to room temperature. Add 1 2 drop 1% Iron (III) chloride test solution. Observe the colour change of the solution 2.Open Rings - 1 ml in test Add 2 - 3 drop 1% NaOH solution, and heat Closed Rings tube under boiling water for 3 - 4 minutes until a clear solution is obtained. Acidify the solution with 3 - 5 drop 2% HCl. Observe the clarity of the solution. (Astragalus membranaceus (Fisch.) Bung) Description of sample species Data / Expected result including color change Aqueous extraction : A1-A5 Ethanolic extraction : B1.1-B1.5; B2, B3.1-B3.2 A. Chemical Reaction Identification - Aqueous Extraction of unknown Chinese Medicines A1. Identification of sugar, polysaccharides and glycosides Reaction Sample Size Procedures 1. Molish 1 ml in test tube Add 1 - 2 drop 10% a-naphthol ethanolic solution and mix, tilt to 45 and add 1 ml concentrated sulfuric acid dropwisely through the test tube wall (2 layers). Observe the colour of the interface of the two layers. A2. Identification of amino acids, polypeptides, and proteins Reaction 1. Biuret reaction Sample Size 1 ml in test tube Procedures Add 1 drop 5% potassium hydroxide solution, mix and add 1% copper sulfate solution with stirring. Observe the colour change of the mixture A3. Identification of Saponins Reaction Sample Size 1. Foaming effect 2 ml in test tube Procedures Shake vigorously for 2 minutes, if large of foam is formed permanently, boil the solution and then shake again. Observe any foam formed again A4. Identification of phenols and tannins Reaction Sample Size Procedures 1. Iron (III) 1 ml in test tube Add a few drop of 1% Iron (III) chloride chloride ethanolic solution. Observe the colour change. 4. Gelatin 1 ml in test tube Add 1 - 2 drop Gelatin-NaCl test Sodium Chloride solution, observe the clarity and the colour of the solution and note if any precipitate formed A5. Identification of Organic Acids Reaction Sample Size 1. pH paper A few drop Procedures Test with pH paper, record the approximate pH value. B. Chemical Reaction Identification - Ethanolic Extraction of unknown Chinese Medicines B1. Take half of filtrate into a 100-ml beaker, evaporate under 95C water bath to about 12.5 ml B2. Take another half of filtrate into a 100-ml beaker, evaporate all ethanol under 95C water bath. Add 10 ml 5% hydrochloric acid, stir thoroughly and then filter, collect the filtrate B3. Add 10 ml ethyl acetate to dissolve the residue, add, and rinse the ethyl acetate solution with 5% sodium hydroxide solution for 2 times (2 3 ml each time). Discard the alkali layer, and rinse the organic layer with deionized water for 1 2 times until the pH of the rinsing water becomes neutral. Evaporate the organic solvent to dryness under steam bath in fume hood and re-dissolve the residue in 15-ml 96% ethanol B1.1 Identification of Tannins Reaction Sample Size 1. Iron (III) 1 ml in test tube chloride Procedures Add a few drop of 1% Iron (III) chloride ethanolic solution. Observe the colour change. Add 1 - 2 drop Gelatin-NaCl test solution, observe the clarity and the colour of the solution and note if any precipitate formed 1 ml in test tube 4. Gelatin - Sodium Chloride B1.2 Identification of Organic Acids Reaction Sample Size 1. pH paper A few drop Procedures Test with pH paper, approximate pH value. record the B1.3 Identification of Flavonoids Reaction Sample Size 1. HCI - Mg 1 ml in test tube powder amount Procedures Add an appropriate of Magnesium powder, mix thoroughly, add 2 - 5 drop concentrated hydrochloric acid. Note if any vigorous reaction occurs and the colour change. B1.4 Identification of anthraquinones Reaction Sample Size 1. Alkali Reaction 1 ml in test tube Procedures Add a few ml of 10% sodium hydroxide solution, note any colour change. Acidify with a few ml of concentrated hydrochloric acid, note any colour change. Add a few drop of 1% magnesium acetate methanolic solution, note the colour change. 1 ml in test tube 2. Magnesium acetate reaction B1.5 Identification of Steroids and Triterpenes Reaction Sample Size Procedures 3. Chloroform - 1 ml in Evaporate under steam bath to dryness, conc. H2SO4 evaporating dish add 1 ml chloroform to dissolve the residue. Transfer the content to a test tube, add 1 ml conc. H2SO4 (2 layers). Observe the colour change of the two layers. B2. Identification of Alkaloids Reaction Sample Size 1. Bismuth 1 ml in test tube Potassium Iodide Procedures Add 1 - 2 drop bismuth potassium iodide test solution, observe any immediate precipitate formed and the colour of the precipitates B3 Identification of Coumarins and Lactones Reaction Sample Size Procedures 1. Iron(III) 1 ml in test Add 2 - 3 drop7% hydroxylamine HCI Hydroxamates tube methanolic test solution and then add 2 - 3 drop 10% KOH methanolic test solution. Heat under boiling water for a few minutes. Cool down to room temperature. Add 1 2 drop 1% Iron (III) chloride test solution. Observe the colour change of the solution 2.Open Rings - 1 ml in test Add 2 - 3 drop 1% NaOH solution, and heat Closed Rings tube under boiling water for 3 - 4 minutes until a clear solution is obtained. Acidify the solution with 3 - 5 drop 2% HCl. Observe the clarity of the solution











