Answered step by step
Verified Expert Solution
Question
1 Approved Answer
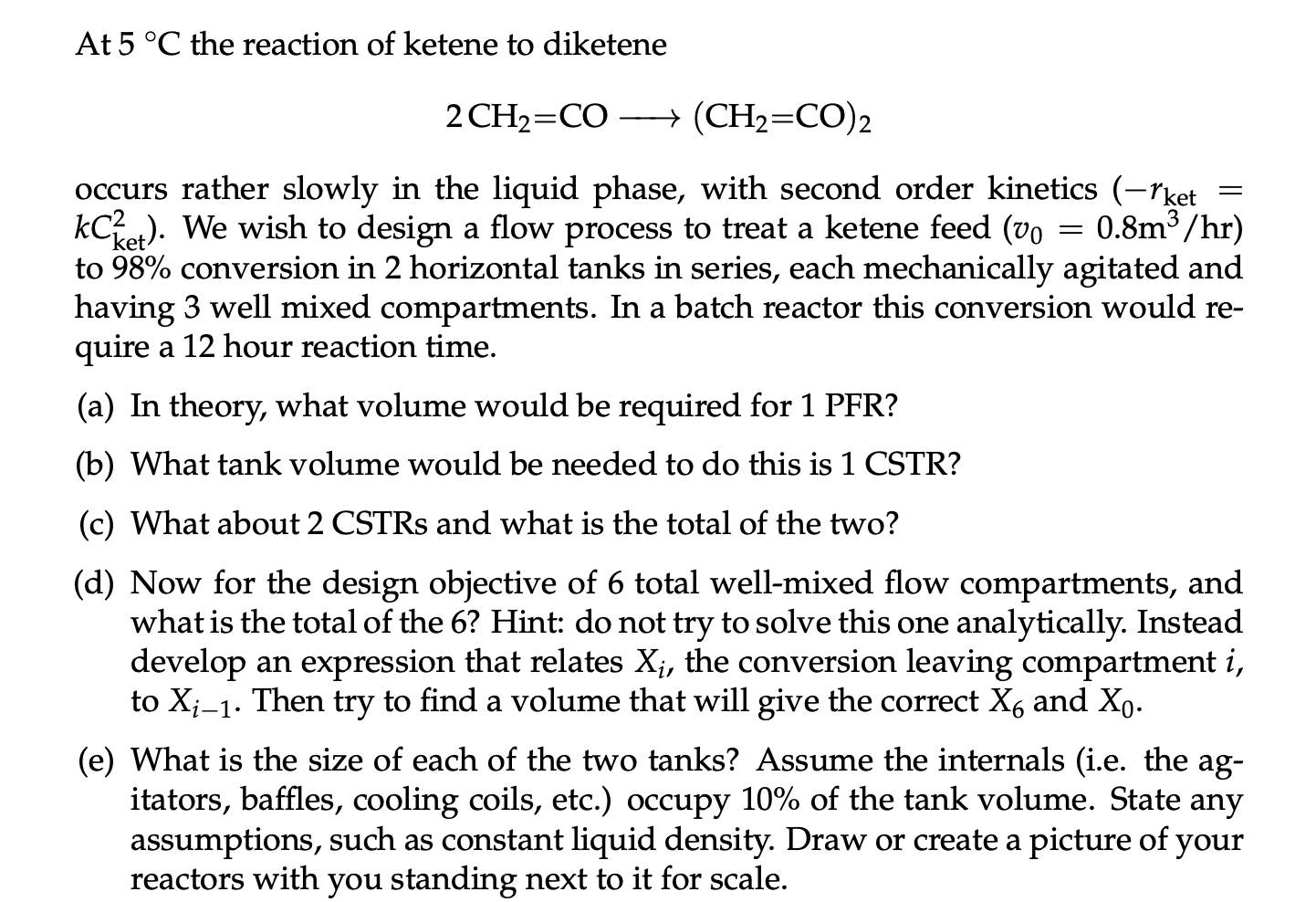
At 5 C the reaction of ketene to diketene 2 ( C H 2 = C O ) ( C H 2 = C O
At the reaction of ketene to diketene
occurs rather slowly in the liquid phase, with second order kinetics
rket We wish to design a flow process to treat a ketene feed
to conversion in horizontal tanks in series, each mechanically agitated and
having well mixed compartments. In a batch reactor this conversion would re
quire a hour reaction time.
a In theory, what volume would be required for PFR
b What tank volume would be needed to do this is CSTR
c What about CSTRs and what is the total of the two?
d Now for the design objective of total wellmixed flow compartments, and
what is the total of the Hint: do not try to solve this one analytically. Instead
develop an expression that relates the conversion leaving compartment
to Then try to find a volume that will give the correct and
e What is the size of each of the two tanks? Assume the internals ie the ag
itators, baffles, cooling coils, etc. occupy of the tank volume. State any
assumptions, such as constant liquid density. Draw or create a picture of your
reactors with you standing next to it for scale.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


