Question
At very low temperatures the molar heat capacity of rock salt varies with temperature according to Debye's T law: C = k where k
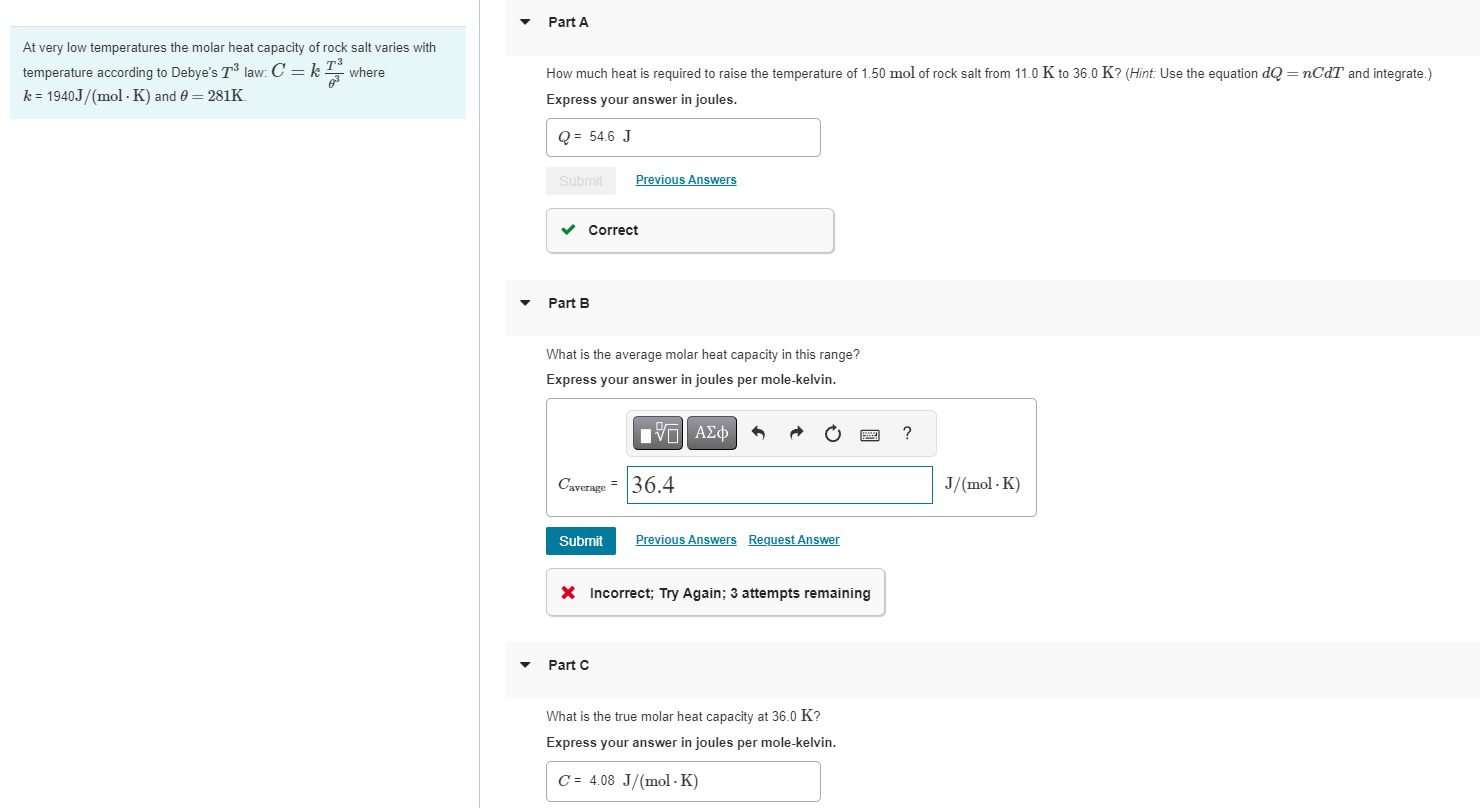
At very low temperatures the molar heat capacity of rock salt varies with temperature according to Debye's T law: C = k where k = 1940J/(mol K) and 0 = 281K. Part A How much heat is required to raise the temperature of 1.50 mol of rock salt from 11.0 K to 36.0 K? (Hint: Use the equation dQ = nCdT and integrate.) Express your answer in joules. Q = 54.6 J Submit Previous Answers Part B Correct What is the average molar heat capacity in this range? Express your answer in joules per mole-kelvin. Caverage 36.4 Submit Previous Answers Request Answer Incorrect; Try Again; 3 attempts remaining Part C What is the true molar heat capacity at 36.0 K? Express your answer in joules per mole-kelvin. C 4.08 J/(molK) ? J/(mol-K)
Step by Step Solution
3.36 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



