Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Azeotropes are mixtures of two or more liquid components that cannot be separated by regular distillation; a well - known azeotrope exists between ethanol and
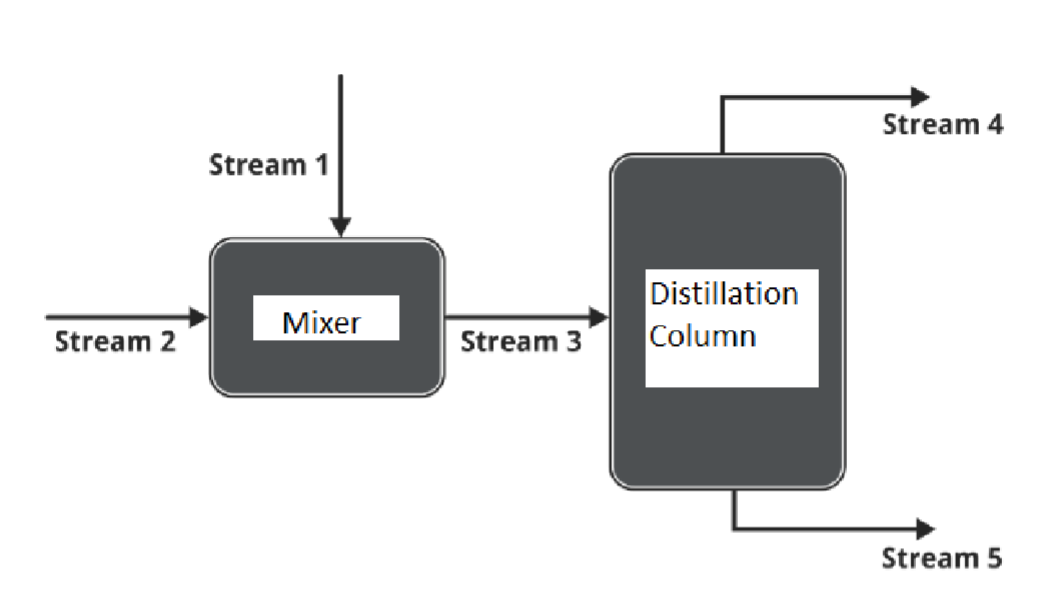
Azeotropes are mixtures of two or more liquid components that cannot be separated by regular distillation; a wellknown azeotrope exists between ethanol and water. One way to break an azeotrope is to add an additional component to mixture and perform additional processing to achieve a higher purity product. An example flowchart of this process using ethanol, water, and acetone is shown below: The following information is known about each stream:
Stream : Pure acetone fed at a rate of Lmin
Stream : A mixture of acetone, ethanol, and water. The component flowrate of water is mol watermin Stream : No information given.
Stream : Total flowrate of molmin with mass acetone and massethanol. It is known that of the ethanol fed to the system exits in this stream.
Stream : A mixture of acetone, ethanol, and water. The stream is molewater. a Perform a degree of freedom analysis for the mixer, the distillation column, and the overall system. Specify an order in which the problem could be solved to
determine all unknowns. b Calculate the rate at which ethanol is fed to the mixer mol ethanolminc Calculate the total molar flow rates in stream and stream molmind Calculate the composition of acetone and ethanol in stream mol fractionseCalculate the total flowrate of the stream entering the distillation column
molmin
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


