Answered step by step
Verified Expert Solution
Question
1 Approved Answer
b) What is the approximate time in minutes to reach steady-state operation? c) Display the entire program code used for your problem solution in Polymath.
b) What is the approximate time in minutes to reach steady-state operation? c) Display the entire program code used for your problem solution in Polymath. Include your name as a comment.
***Use Polymath to solve this, and please show steps!***
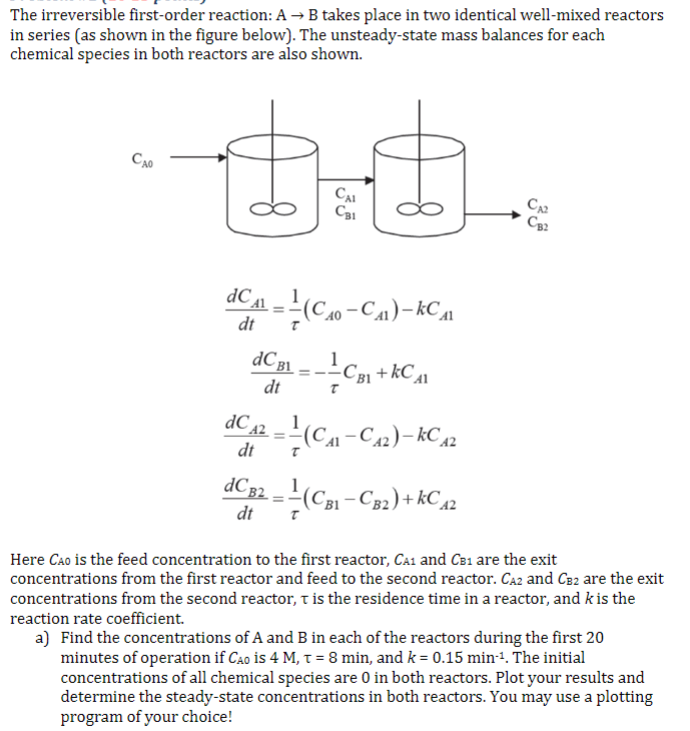
The irreversible first-order reaction: A - B takes place in two identical well-mixed reactors in series (as shown in the figure below). The unsteady-state mass balances for each chemical species in both reactors are also shown. D. CAL C31 C32 CA1 dt dCBL dt dC (C:00-Ca)- kC 1 AC 31+kCa C (C1-C-2)- kC 12 dC 32 = ?(C31-C82) + kC 12 dC dt A2 dt Here Cao is the feed concentration to the first reactor, CA and CB1 are the exit concentrations from the first reactor and feed to the second reactor. CA2 and Cez are the exit concentrations from the second reactor, t is the residence time in a reactor, and k is the reaction rate coefficient. a) Find the concentrations of A and B in each of the reactors during the first 20 minutes of operation if Cao is 4 M, T = 8 min, and k = 0.15 min-1. The initial concentrations of all chemical species are 0 in both reactors. Plot your results and determine the steady-state concentrations in both reactors. You may use a plotting program of your choiceStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


