Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Based on the information i attached can you please answer the discussion questions to the best of your ability. indicator Lab. #6 Spectrophotometric Determination of
Based on the information i attached can you please answer the discussion questions to the best of your ability. 
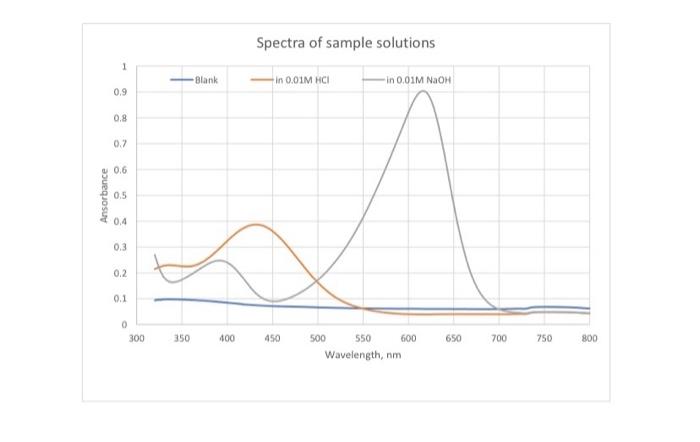
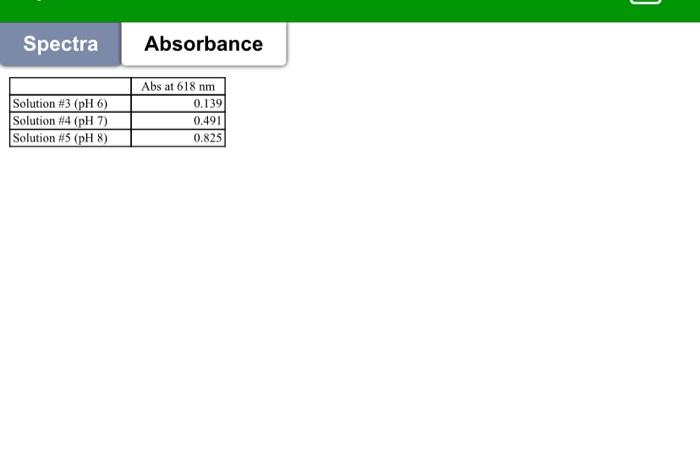

indicator Lab. #6 Spectrophotometric Determination of the K. of a pH indicator INTRODUCTION An acid-base indicator exists in two forms that show different colors, i.e. acidic form (Hin) and basic form (In'); Hins H + In K. = [H"] [in]/[Hin! Color 1 Color 2 At low pH the indicator exists completely in the Hin form and likewise, at high pH the indicator exists completely in the inform these two solutions are used to determine maande for both Hin and in). As pH changes, the relative concentrations of each form will change in accordance with the K, of the indicator. Therefore, by monitoring the change in absorbance with a change in pH, it is possible to extract a value for the K, of the indicator. PROCEDURE 1. Prepare five clean and dry 10-ml volumetric flasks and label them as 01 through 5. Add (2.00 ml each) 0.01M HCl solution to flask #1,0.01M NaOH solution to flask #2. pH 6.00 buffer solution to flask #3, pH 7.00 buffer solution to flask #4, pH 8.00 buffer solution to flask 45. Add 0.20 ml of a 2.50 x 10 M bromothymol blue solution to each of the five flasks. Dilute each flask with water to mark. Cand indicator 2. Using the scanning spectrophotometer, obtain and record spectra between 320 and 800 nm of solutions #1 and #2 against water (baseline). (Your instructor will show you how to use the instrument) 3. Measure absorbance from solutions #3, #4, and #5 at wavelength 618nm. acidic form basic form HI+ HO - HO+ + I Spectra of sample solutions 1 Blank in 0.01M MCI in 0.01M NaOH 0.9 0.8 0.2 0.6 Ansorbance 0.5 0,4 0.3 0.2 0.1 0 300 350 400 450 650 700 250 800 500 550 600 Wavelength, nm Spectra Absorbance Solution #3 (pH 6) Solution #4 (pH 7) Solution #5 (pH 8) Abs at 618 nm 0.139 0.491 0.825 Cher DATA PRESENTAT/CALCULATION 1. Make ONE graph showing the baseline and the spectra of both Hin and in (obtained from Solution #1 and 2, respectively). 2. Tabulate the absorbance at 620nm (corrected from the baseline) of each of the 5 solutions together with the pH values of respective solutions. 3. From each of the two spectra and indicator concentration, determine the for both Hin and in, and values for both Hin and in at both wavelengths 4. Using equation: Abs & C + c26 t... and the absorbance data, calculate (in) and [Hin) in each of the three buffered solutions, le solutions #3, #4, and #5. Place the results in the above table (made in step #2) 5. Calculate the K. of bromothymol blue from the concentration results obtained from each of the three solutions #3, #4, and #5. Calculate the average of Kvalues. Place the results in the above table (made in step #2) DISCUSSION 1. Write the structure of the indicator studied 2. Compare the land e values you measured with that in literature. Explain for the discrepancy (if any) between the two 3. Compare the value you measured with that reported in literature. Explain for the discrepancy (if any) 4. Explain why this method is not suitable for the determination of the Kof phenolphthalein 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


